Figure 2. Immunotherapy Adoption Before and After US Food and Drug Administration (FDA) Approval by Practice Type.
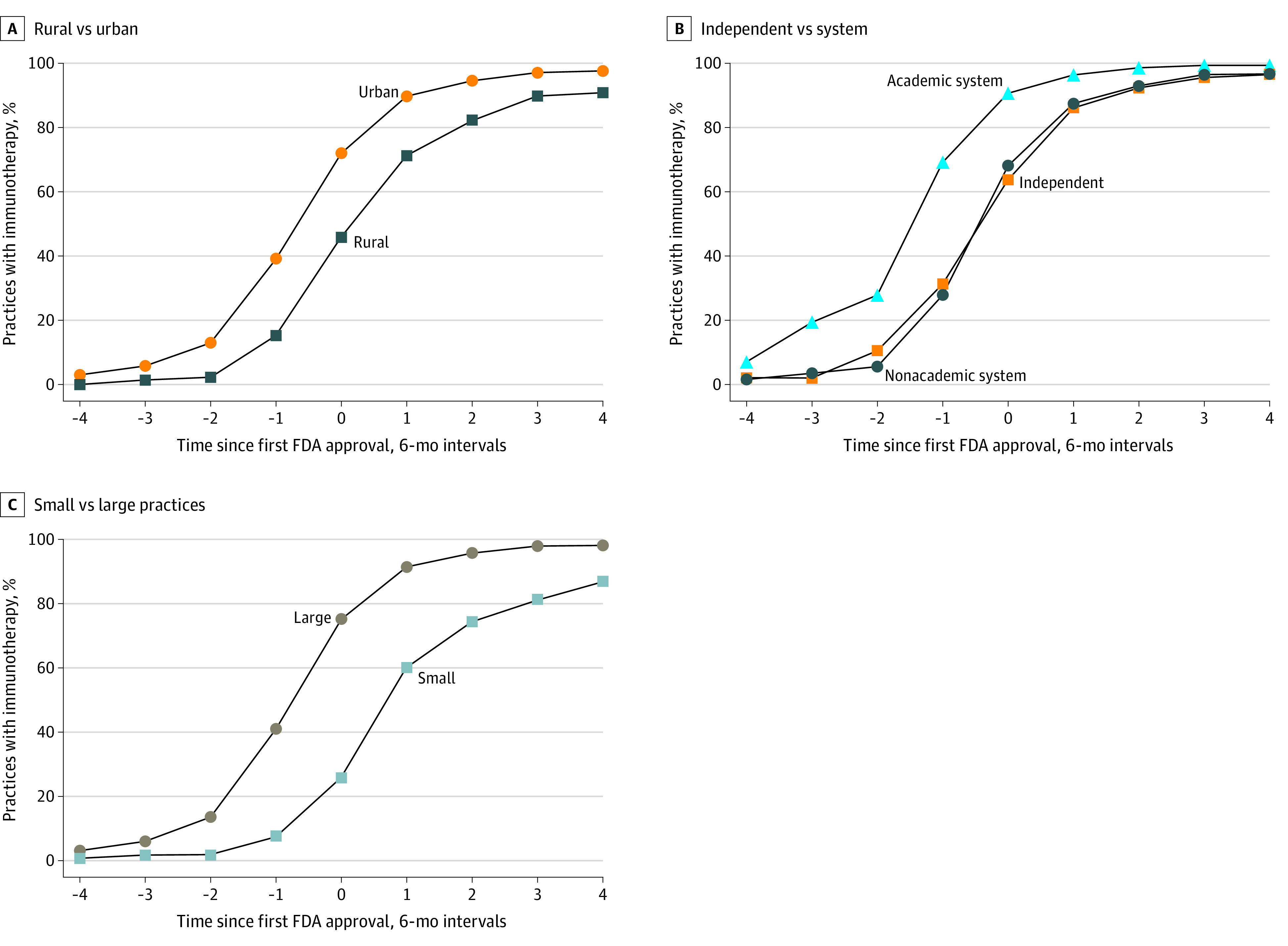
Immunotherapy adoption rates are plotted by practice type before and after FDA approval. Because some immunotherapy indications were approved near the end of the study period in 2017, not all cancer types are included in each time period (eg, immunotherapy was approved for head and neck cancer in 2016, allowing for 1 year of postapproval observation). C, Large practices included 6 or more physicians; small practices included 1 to 5 physicians.
