Figure. Characteristics of Predicate Medical Devices Over Time.
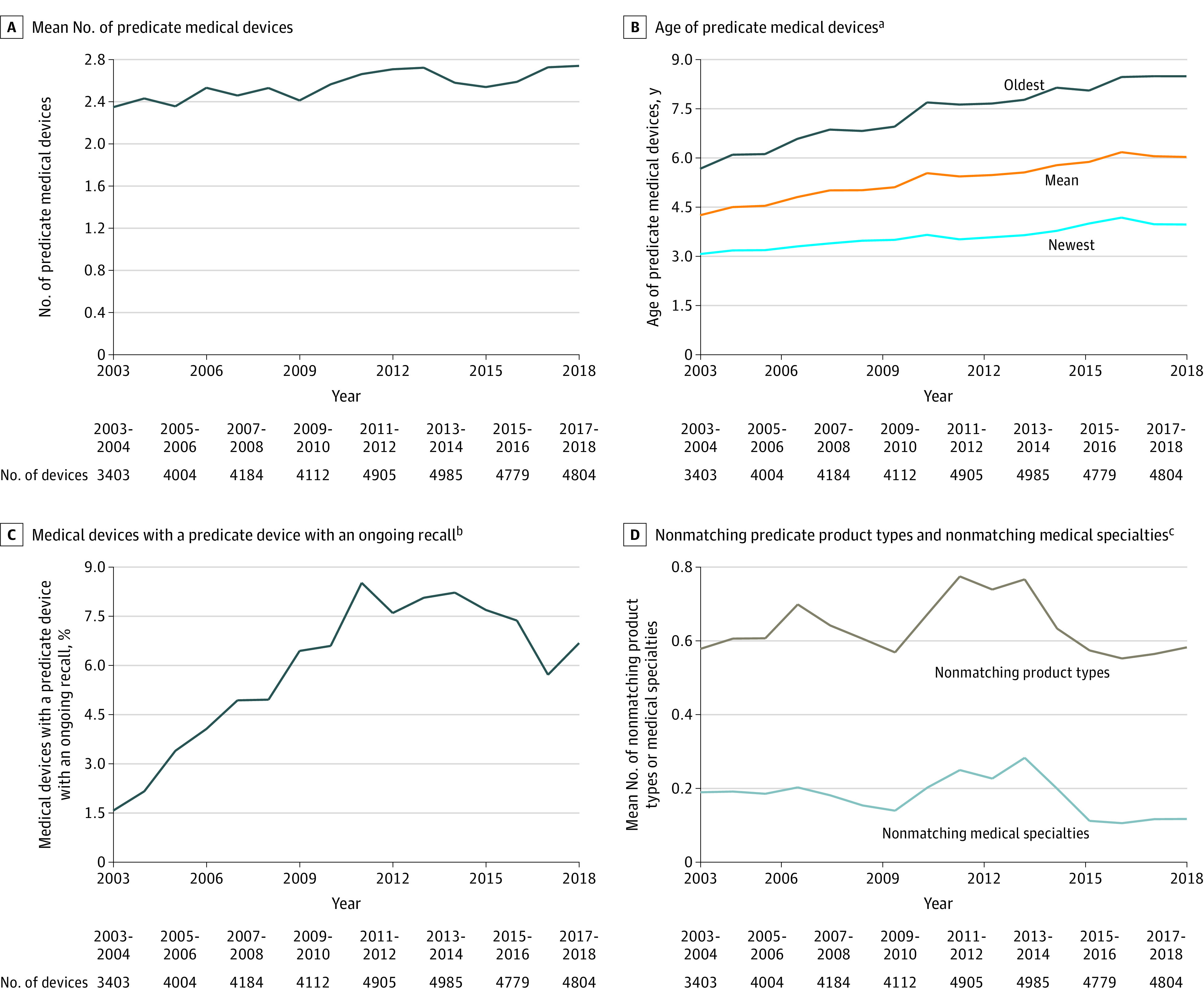
aDefined as the number of years between US Food and Drug Administration (FDA) clearance of a predicate medical device and FDA clearance of the applicant device.
bDefined as a class I or a class II ongoing recall for the predicate medical devices classified by the FDA but not yet terminated (resolved) at the time of FDA clearance for the applicant device. A class I recall indicates there is potential for serious patient harm or death. A class II recall indicates there is potential for temporary or reversible patient harm or a slight chance of serious patient harm or death.
cNonmatching product types refer to the number of unique product types of the predicate medical device that do not match the product types of the applicant device. The product types are FDA-assigned identifiers to describe the generic function of a medical device (additional information appears in the study sample subsection of the Methods section). Nonmatching medical specialties refer to the number of unique medical specialties of the predicate medical device that do not match the medical specialties of the applicant device. There are 19 FDA medical specialty review panels that are responsible for reviewing a medical device. The medical specialty review panels are assigned based on a medical device’s product type.
