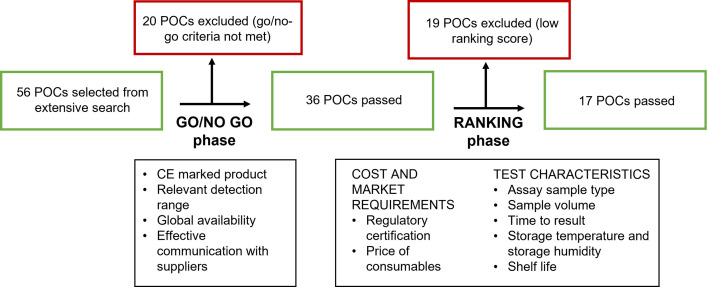
Fig 1. Flowchart for tests selection.
Go/no go criteria included both objective and subjective criteria: Whether the test was CE-marked, relevant detection range (from 10 to 100 mg/L CRP for a quantitative test, and one cut-off value of ≥20 mg/L for qualitative and semi-quantitative tests), worldwide distribution, and responsiveness of the manufacturer. Ranking criteria included: Additional regulatory certification (higher score for US Food and Drug Administration-approved products); cost of consumables (lower score for test price >10 USD, higher score for test price <1 USD); assay sample type (lower score for tests requiring serum samples only, higher score for tests that can be used with capillary whole blood, venous blood, or plasma); sample volume (lower score for tests requiring a volume of >50 μL, higher score for tests requiring a volume of <10 μL); time to result (lower score for tests requiring >20 min, higher score for tests requiring <5 min); storage temperature (lower score for tests requiring <4°C, higher score for tests stable up to 40°C); shelf-life (lower score for tests stable for <12 months, higher score for tests stable for >24 months).