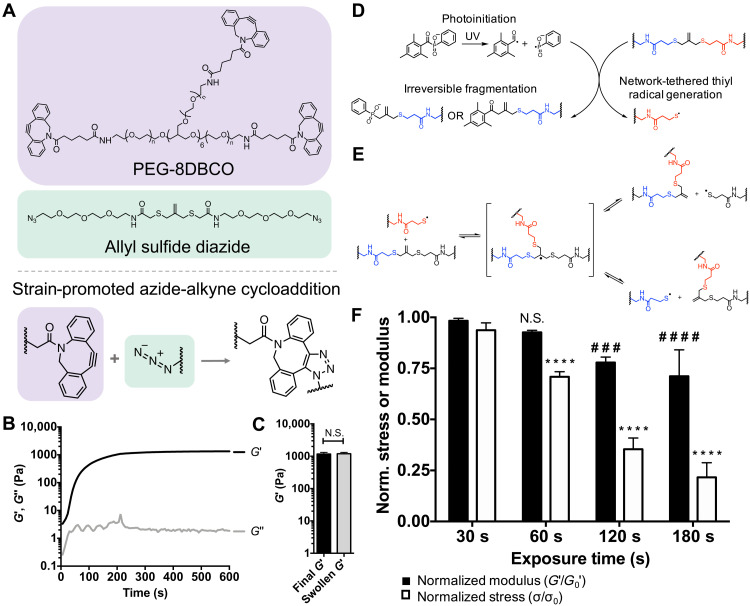
Fig. 1. Hydrogel formation and photoinduced viscoelastic response.
(A) Chemical structures of 8-arm poly(ethylene glycol) dibenzylcyclooctyne (PEG-8DBCO) and allyl sulfide diazide, which react through strain-promoted azide-alkyne cycloaddition (SPAAC) to form a hydrogel. (B) The shear storage modulus (G′; black) increased during gelation, reaching 90% of the plateau values at 236 ± 12 s, while the shear loss modulus (G″; gray) remains constant. Rheological plot shows representative trace. (C) Hydrogels achieved a final storage modulus of 1170 ± 150 Pa upon gelation, with no significant difference upon equilibration. Significance was determined by t test, N = 4 gels, means ± SD. (D) Incident ultraviolet (UV) light generates radicals from soluble lithium phenyl-2,4,6-trimethylbenzoylphosphinate (LAP) that react with allyl sulfide cross-links to generate network tethered thiyl radical species through irreversible fragmentation. (E) Network-tethered thiyl radical species then react with other allyl sulfides in an exchange reaction, regenerating an allyl sulfide cross-link and a network-tethered thiyl radical species, which can then participate in other exchange reactions. (F) The normalized modulus (black) and stress (white) of allyl sulfide cross-linked hydrogels decreased following increasing exposure times 405 nm light at 10 mW cm−2. Each sample was normalized to the modulus or stress value before irradiation. Significance was determined by two-way analysis of variance (ANOVA) with Tukey’s multiple comparison test; number sign (#) denotes significance compared to a 30-s normalized modulus condition, and asterisk (*) denotes significance compared to a 30-s normalized stress condition; ###P < 0.001; #### and ****P < 0.0001; N = 3 gels per condition, means ± SD. N.S., not significant.