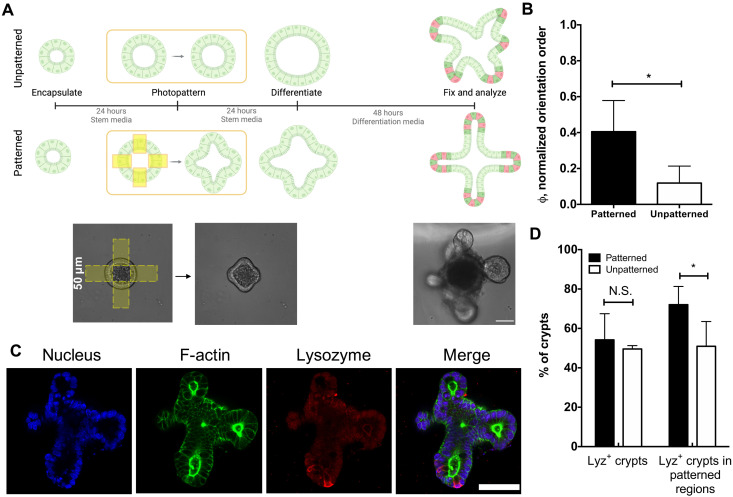
Fig. 4. Photopatterned changes in epithelial curvature template crypt formation.
(A) Organoids were encapsulated into hydrogels and allowed to expand for 24 hours before applying photopatterns. The “unpatterned” condition did not receive any light dose. After 24 hours, patterned and unpatterned organoids were exposed to differentiation conditions, and crypt growth was assessed 48 hours later. Scale bar, 50 μm. (B) The angles of crypt formation were used to generate an orientation order parameter, which is higher for patterned organoids (black) compared to unpatterned (white) organoids, indicating that crypt formation is directed and templated by patterned epithelial shape. Significance was determined by t test; *P < 0.05; N = 4 and 3 gels for the patterned and unpatterned conditions, respectively. Bars denote average ± SD. (C) Immunostaining of differentiated organoids for nuclei (blue), F-actin (green), and lysozyme (red), a marker for Paneth cells, revealed Paneth cells confined to crypt ends. Scale bar, 50 μm. (D) Analysis of lysozyme distribution in patterned (black) and unpatterned (white) organoids revealed that 54 ± 13% and 49 ± 2% of all crypts contained lysozyme-positive cells in patterned and unpatterned organoids, respectively, while 72 ± 9% and 50 ± 12% of crypts in patterned regions contained lysozyme-positive cells in patterned and unpatterned organoids, respectively. Significance was determined by one-way ANOVA with Tukey’s multiple comparison test; *P < 0.05; N = 4 gels. Bars denote average ± SD.