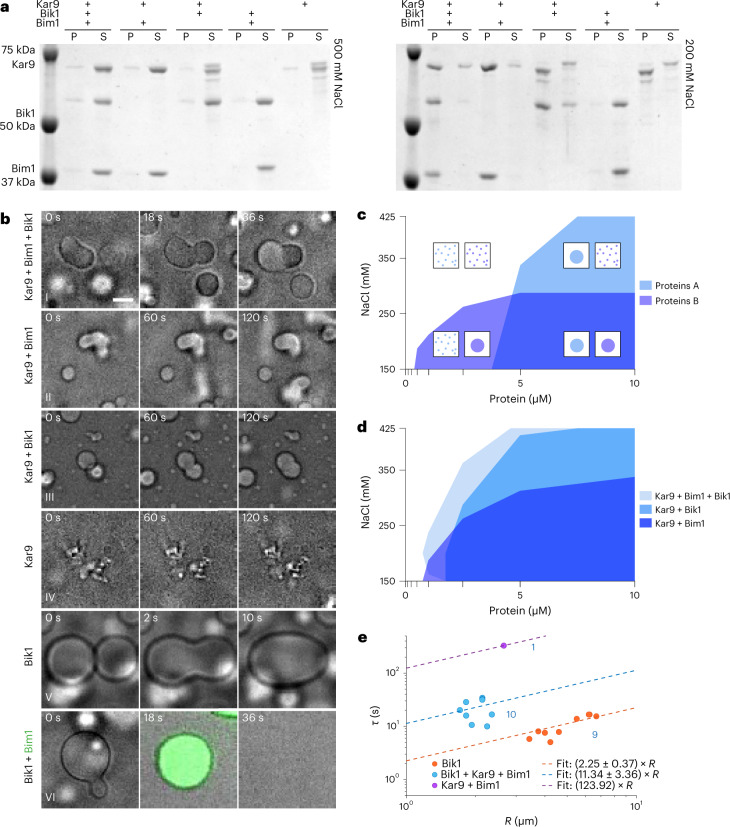
Fig. 1. Characterization of Kar9, Bim1 and Bik1 phase separation in vitro.
a, Pelleting assays of Kar9 and mixtures with Bim1 and Bik1 (10 μM each) analysed by Coomassie-stained SDS–PAGE at 500 mM (left) or 200 mM (right) NaCl. P, pellet; S, supernatant. For partitioning between light and dense phases, see Extended Data Fig. 2a. b, Time-lapse phase contrast micrographs of mixtures of proteins as indicated on the left. For Bik1 + Bim1, Bik1 droplets were pre-formed and then Atto-488-NTA-labelled Bim1 was added. Scale bar, 3 μm. See also Supplementary Movies 1–6. For additional FRAP data, see Extended Data Fig. 1. c, Schematic 2D superimposed phase diagram for separate experiments with imaginary proteins or protein mixtures A and B to compare their phase behaviour. Ticks on the axes indicate tested conditions, coloured areas the extrapolated regions with phase separation. Illustrations describe the local condensation states. d, Superimposed phase diagrams of equimolar mixtures of Kar9 + Bim1 + Bik1 (light blue), Kar9 + Bik1 (intermediate blue) and Kar9 + Bim1 (dark blue) obtained at different protein and NaCl concentrations. Protein concentration is the concentration of each protein in the equimolar mixture. Single experiment. For titration of Kar9 into constant Bim1 and Bik1, see Extended Data Fig. 2b. e, Droplet fusion time τ as a function of droplet radius R based on phase contrast microscopy movies (b) on a log–log plot. Dots indicate measurements; lines indicate linear fits to the data with reported slopes of τ as a function of R, blue values indicate n = number of droplets, single experiment. Details in Methods and Extended Data Fig. 10. Source numerical data and unprocessed gels available in source data.