Figure 1.
Generation of cochlear organoids from single CPCs in the chemical-defined culture system
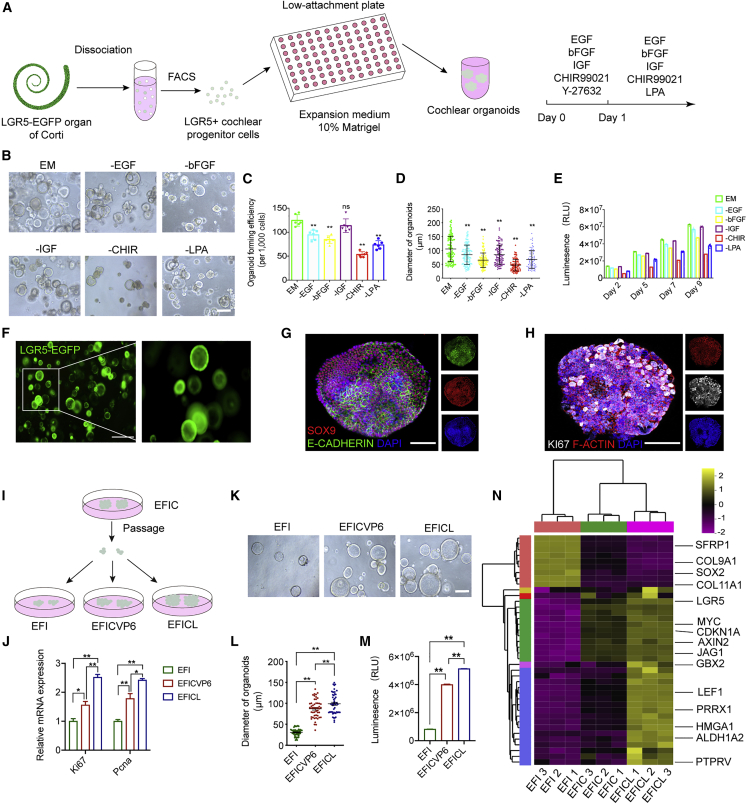
(A) A schematic of the experimental strategy for generating cochlear organoids from CPCs.
(B) Representative bright-field images of cultured organoids (P0) from single CPCs under the indicated conditions. Scale bar, 150 μm.
(C) Quantifying organoid-forming efficiency under the indicated conditions (n = 6 independent experiments). The data are presented as means ± SEM. ∗∗p < 0.01, nsp ≥ 0.05 (one-way ANOVA followed by Tukey’s multiple comparisons test).
(D) Quantification of organoid size in different media. Each dot represents an organoid. 96–101 organoids at each condition, three independent experiments. The data are presented as means ± SEM. ∗∗p < 0.01 (one-way ANOVA followed by Tukey’s multiple comparisons test).
(E) Quantification of organoid viability cultured in expansion medium or medium where one specific component was removed (n = 5 independent experiments).
(F) Representative fluorescence images showing the cochlear progenitor cell marker LGR5 expression as indicated by EGFP in P0 organoids (day 8). Scale bar, 500 μm.
(G) Immunofluorescence analysis of P0 organoids at day 8 showing the expression of stem cell marker SOX9 and the epithelial cell marker E-CADHERIN. Scale bar, 50 μm.
(H) Immunofluorescence analysis of organoids at day 8 showing the expression of the proliferative cell marker KI67 and the cytoskeleton protein F-ACTIN. Scale bar, 50 μm.
(I) A schematic of the experimental strategy of comparing the pro-proliferative effect of the three different conditions. EFI refers to EGF, bFGF, and IGF; EFICVP6 refers to EGF, bFGF, IGF, CHIR, VPA, pVc., and 616452; EFICL refers to EGF, bFGF, IGF, CHIR, and LPA.
(J) Real-time PCR analysis showed the relative expression of proliferation-related genes. Results were normalized to GAPDH in the same sample and then normalized to the expansion group (n = 3 independent experiments). The data are presented as means ± SEM. ∗p < 0.05, ∗∗p < 0.01 (one-way ANOVA followed by Tukey’s multiple comparisons test).
(K) Representative bright-field images of cultured organoids under the indicated conditions. Scale bar, 100 μm.
(L) Quantification of organoid size in different media. 50 organoids at each condition, three independent experiments. The data are presented as means ± SEM. ∗∗p < 0.01 (one-way ANOVA followed by Tukey’s multiple comparisons test).
(M) Luminescent cell viability assay measuring CPCs cultured in the indicated conditions (n = 3 independent experiments). The data are presented as means ± SEM. ∗∗p < 0.01 (one-way ANOVA followed by Tukey’s multiple comparisons test).
(N) Heatmap of inner ear gene expression determined by mRNA sequencing of organoids cultured in the three conditions: essential expansion medium alone, + CHIR, and + CHIR + LPA (n = 3 independent experiments).