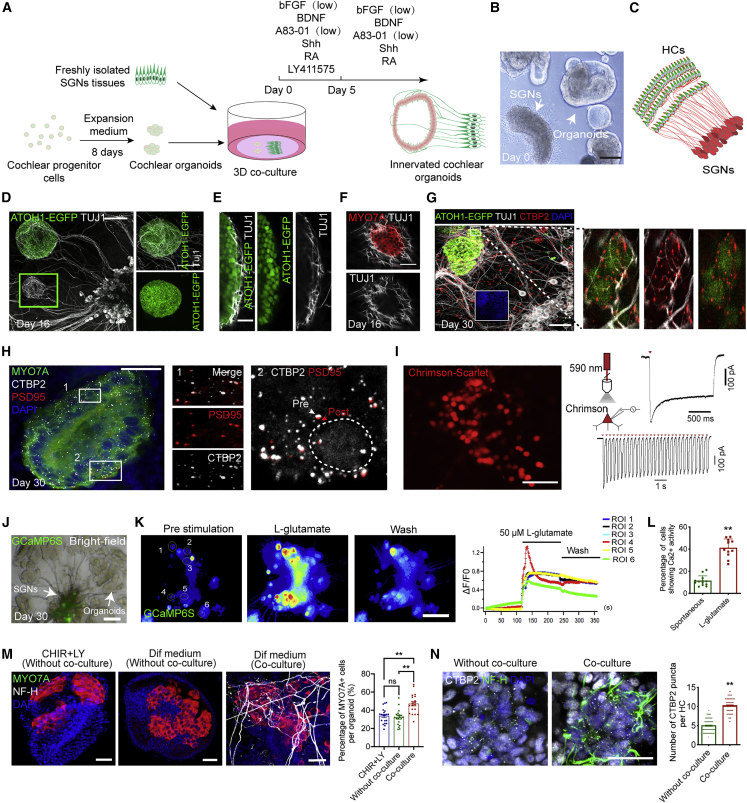
Figure 3.
Generating SGN-innervated cochlear organoids with functional auditory circuits
(A) A schematic of the experimental strategy for generating SGN-innervated cochlear organoids by 3D co-culture of cochlear organoids and SGNs.
(B) Bright-field image of co-cultured organoids and SGNs at day 0. Scale bar, 100 μm.
(C) Schematic representation of the peripheral auditory circuit where HCs in the cochlear epithelium connect with SGNs.
(D) Fluorescence images of the co-culture system showed the innervation of ATOH1+ organoid HCs by tracts from TUJ1+ SGNs at day 16. Scale bar, 100 μm.
(E) Higher magnification of the HC region shows the TUJ1+ SGN neurites in contact with ATOH1+ HCs. Scale bar, 25 μm.
(F) Immunofluorescence analysis showed that TUJ1+ neurites surrounded the MYO7A+ HCs. Scale bar, 25 μm.
(G) Representative fluorescence images of co-cultures at day 30 showing TUJ1+ SGN axon projections into ATOH1+ HCs with CTBP2+ presynaptic vesicles (left) and higher magnification of the SGN terminals of the selected region in contact with CTBP2+ presynaptic vesicles (right). Scale bar, 50 μm.
(H) Representative fluorescence images showing the presence of CTBP2+ presynaptic vesicles and PSD95+ postsynaptic vesicles in the MYO7A+ HCs (left). Scale bar, 25 μm. Higher magnification of the HC region showing the CTBP2+ presynaptic vesicles co-localized with PSD95+ postsynaptic vesicles (right). The circle represents the localization of HCs.
(I) Fluorescence microscopy images of SGNs transfected with adeno-associated virus (AAV) DJ-Chrimson-Scarlet. Scale bar, 50 μm. The right representative images showed the red light-induced spiking traces of SGNs at 5 mW/mm2.
(J) Bright-field and fluorescence microscopy image of co-cultured organoids and SGNs at day 30. SGNs transfected with AAV 2/9-hSyn-GCaMP6S. Scale bar, 100 μm.
(K) Representative calcium imaging of SGNs in co-cultures at day 30. Scale bar, 100 μm. The single-cell tracings of the region of interest (ROI). Time is shown in seconds (s).
(L) Quantification of the percentage of firing SGNs on day 30 of SGN-innervated cochlear organoids under spontaneous conditions or after L-glutamate treatment (11–13 co-cultures at each condition, four independent experiments). The data are presented as means ± SEM. ∗∗p < 0.01 (t test).
(M) Immunofluorescence analysis of organoids at day 30 stained with MYO7A and neurofilament-H (NF-H) in the three different culture conditions. Scale bar, 25 μm. Quantification of the percentage of MYO7A+ cells per organoid. 20 organoids at each condition, three independent experiments. The data are presented as means ± SEM. ∗∗p < 0.01, nsp ≥ 0.05 (one-way ANOVA followed by Tukey’s multiple comparisons test).
(N) Immunofluorescence analysis of organoids at day 30 stained with CTBP2 and NF-H in the three different culture conditions. Quantification of the number of CTBP2+ presynaptic vesicles per HC from single cultured organoids and co-cultures at day 30. 100 HCs from 8–12 organoids at each condition, three independent experiments. The data are presented as means ± SEM. ∗∗p < 0.01 (t test).