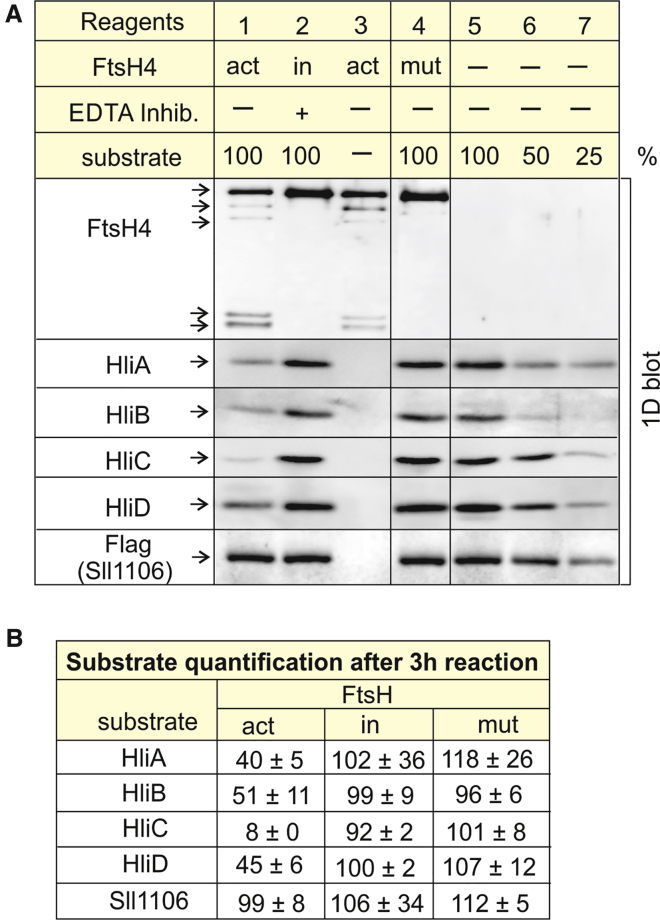
Figure 4.
FtsH4 proteolytic assay in vitro.
(A) Hlips, Sll1106, and FtsH4 variants were isolated from membranes of Synechocystis strains expressing His-tagged (FtsHs and Hlips) or Flag-tagged (Sll1106) protein derivatives as described in methods. Reagents used in individual reactions 1–7 are presented in the table above the blot. At the start of the reaction, samples 1–4 contained 0.3 μg of FtsH4 protein in total. Samples 1/2 and 4/5 contained 0.2 μg of the substrate (Hlips or Sll1106, respectively), corresponding to 100% in calibration. Samples 1–3 contained the active variant of FtsH4 (act). Sample 2 was supplemented with protease inhibitor cocktail containing EDTA to inhibit activity of the FtsH protease (in). Sample 4 contained inactive FtsH4 variant (mut). Composition of the reaction buffer: 25 mM MES (pH 6.5), 5% glycerol, 2 mM Mg2+, 2 mM Ca2+, 0.4 μM Zn2+, and 3 mM ATP. All samples were incubated for 3 h at 37°C. After incubation, the samples were denatured by 1% SDS and analyzed by 1D SDS–PAGE. The protein bands were electroblotted to polyvinylidene fluoride; the presence of substrates and FtsH4 was detected by specific antibodies, and Sll1106-FLAG was detected by FLAG antibody.
(B) Substrate quantification: the percentage of the substrate in a particular reaction sample relates to the initial substrate amount (100%); values are means of three measurements of three independent reactions ± standard deviation. Quantification of bands was performed with ImageQuant TL software (GE Healthcare, Uppsala, Sweden).