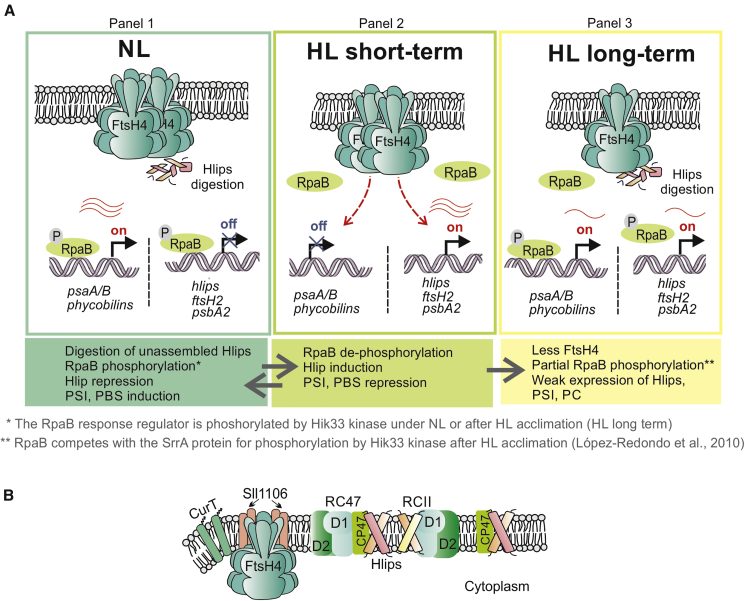
Figure 7.
Model for FtsH4 role in acclimation to HL and its localization in the TM.
(A) The role of FtsH4 in the transcriptional and proteolytic regulation of Hlips under various light conditions. Under NL (panel 1), Hik33-phosphorylated RpaB represses expression of HL-inducible genes and activates expression of PSI and phycobiliprotein (PBS) genes. Unnecessary Hlips are degraded by FtsH4. After a shift to high light (HL; panel 2), Hik33 is transiently switched to the phosphatase conformation, leading to RpaB dephosphorylation and its release from DNA binding. This causes derepression of HL-inducible genes and repression of PSI/PBS genes. The presence of FtsH4 stimulates Hlip induction and at the same time PSI/PBS repression. FtsH4 might regulate the level of RpaB response regulator or possibly some σ factor from group 2, such as SigD (Sll2012) or SigE (Sll1689) (Srivastava et al., 2020). After long-term HL exposure (acclimation to HL, panel 3), Hik33 regains kinase activity but preferentially phosphorylates SrrA (a product of HL-inducible genes), and RpaB is only partially phosphorylated. Therefore, both Hlips and PSI/PBS are expressed, but weakly. Unnecessary Hlips are degraded by FtsH4. The red wavy lines represent mRNA, and their number indicates the amount of transcript.
(B) Co-localization of the FtsH4 protease with proteins connected to PSII assembly. Proteins co-purified with FtsH4 are assumed to directly interact or co-localize with the protease. These were Sll1106, CurT (Slr0483), and several subunits of PSII, preferentially CP47. FtsH4, together with Sll1106 and CurT, was also found to be associated with the D1/D2 assembly intermediates (Knoppová et al., 2022). RC47 and RCII: PSII intermediates lacking CP43 or both CP47 and CP43 core antennas, respectively.