Abstract
In different organs and tissues, the lymphatic system serves as a drainage system for interstitial fluid and is useful for removing substances that would otherwise accumulate in the interstitium. In the brain, which lacks lymphatic circulation, the drainage and cleaning function is performed by the glymphatic system, called so for its dependence on glial cells and its similar function to that of the lymphatic system. In the present article, we define glymphatic insufficiency as the inability of the glymphatic system to properly perform the brain cleaning function. Furthermore, we propose that corpora amylacea or wasteosomes, which are protective structures that act as waste containers and accumulate waste products, are, in fact, a manifestation of chronic glymphatic insufficiency. Assuming this premise, we provide an explanation that coherently links the formation, distribution, structure, and function of these bodies in the human brain. Moreover, we open up new perspectives in the study of the glymphatic system since wasteosomes can provide information about which variables have the greatest impact on the glymphatic system and which diseases occur with chronic glymphatic insufficiency. For example, based on the presence of wasteosomes, it seems that aging, sleep disorders, and cerebrovascular pathologies have the highest impact on the glymphatic system, whereas neurodegenerative diseases have a more limited impact. Furthermore, as glymphatic insufficiency is a risk factor for neurodegenerative diseases, information provided by wasteosomes could help to define the strategies and actions that can prevent glymphatic disruptions, thus limiting the risk of developing neurodegenerative diseases.
Keywords: glymphatic system, corpora amylacea, wasteosomes, brain
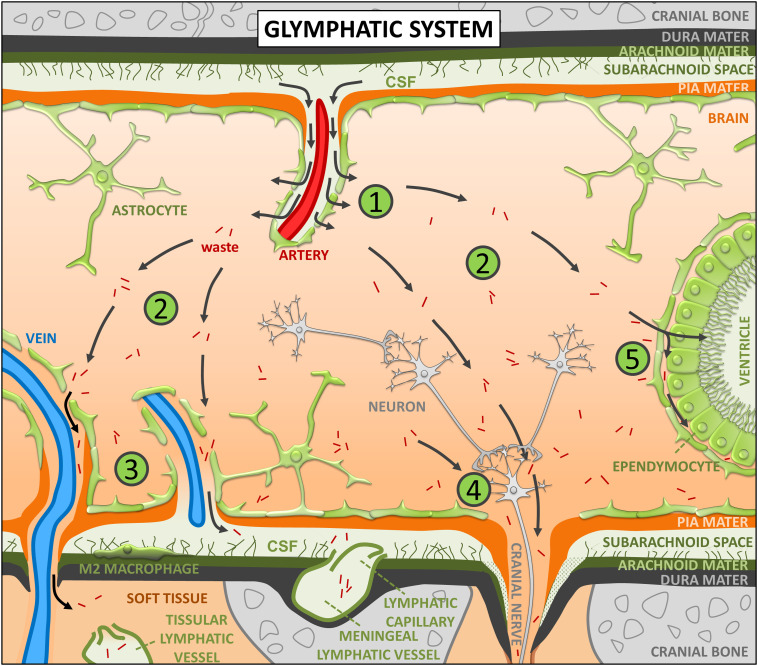
In different organs and tissues, the lymphatic system serves as a drainage system for interstitial fluid (ISF) and is useful for removing substances that would otherwise accumulate in the interstitium. In the brain, which lacks lymphatic circulation, the drainage function is performed by the newly discovered glymphatic system, called so for its dependence on glial water channels and its similar function to that of the lymphatic system (1, 2). Briefly, the glymphatic system involves the entry of cerebrospinal fluid (CSF) from the subarachnoid space into the periarterial spaces, principally those of the three main cerebral arteries (anterior, middle, and posterior cerebral arteries), and then its movement along the periarterial spaces propelled by the pulsatility of the arterial walls (1, 3–5). Later, aquaporin-4 (AQP4) water channels that are abundantly present at the vascular astrocytic end feet facilitate the displacement of water from the periarterial spaces to the brain parenchyma, where it mixes with brain ISF (6). Moving along an anatomical pathway that is guided in some cases by the white matter tracts (2, 7), fresh ISF flows across the brain parenchyma toward the perivenous spaces, principally those of the internal cerebral vein of the deep venous system and the inferior anastomotic vein of Labbé of the superficial venous system (1). ISF progresses along these perivenous spaces and leaves the nervous system, most of it bypassing the CSF of the subarachnoid space (1, 2, 8) and eventually draining through the traditional lymphatic vessels located in the soft tissue surrounding the skull or through the meningeal lymphatic vessels (2, 9). Several additional pathways that ISF uses to exit the nervous system include the cranial and spinal nerves, as well as both the subependymal and transependymal routes (2, 7, 10, 11). As a result, the glymphatic system drags away substances that would otherwise accumulate in the brain parenchyma, therefore constituting an important brain cleaning system (12). All these processes are illustrated in Fig. 1.
Fig. 1.
Brief description of the glymphatic system. (1) The glymphatic system involves the entry of CSF from the subarachnoid space into the periarterial spaces, its movement along these spaces propelled by the pulsatility of the arterial walls, and the displacement of water from the periarterial spaces to the brain parenchyma, where it mixes with brain ISF. (2) Fresh ISF flows across the brain parenchyma, moving along an anatomical pathway that is guided in some cases by the white matter tracts, and dragging away substances that would otherwise accumulate in the parenchyma. (3) Some of the ISF leaves the brain through the perivenous spaces (principally those of the large-caliber ventral veins and some superficial veins), most of it bypassing the CSF of the subarachnoid space. (4) ISF can also drain through the cranial and spinal nerves. (5) The subependymal and transependymal routes are additional pathways that ISF uses to exit the nervous system. As a result, the glymphatic system constitutes an important brain cleaning system. See text for details.
In accordance with this and from a theoretical point of view, we can define glymphatic insufficiency as the inability of the glymphatic system to properly perform the brain cleaning function. Again, from a theoretical point of view, this insufficiency can be acute if it occurs abruptly and suddenly, or it can be chronic if it is maintained over time or lasts a while. Glymphatic insufficiency, whether acute or chronic, can occur because of a failure in the system itself or by overproduction of waste substances that exceeds the cleaning capacity of the system. Moreover, a combination of the two events cannot be excluded. Either way, if glymphatic insufficiency occurs, the result will be the accumulation of waste substances in the brain parenchyma.
In this article, we propose that corpora amylacea or wasteosomes, which are protective bodies that act as waste containers and accumulate brain waste products (13–15), are in fact a manifestation of glymphatic insufficiency, specifically chronic glymphatic insufficiency. We will explain below the different elements that led us to propose this hypothesis and how this allows some conclusions to be drawn regarding the functioning or malfunctioning of the glymphatic system in different situations or diseases.
Wasteosomes are composed of a polyglucosan structure that retains or accumulates waste elements (13–17). The polyglucosan structure is composed of aggregates of polymeric chains of hexoses, mainly glucose, which are more similar to the amylopectin of vegetable starch than to animal glycogen (13, 16, 18, 19). This similarity to starch led to these bodies being named corpora amylacea, a Latin term for "starch-like bodies" (19, 20). Regarding the waste elements or elements that are associated with these polyglucosan structures, a great variety has been described, including components of neuronal, astrocytic, or oligodendrocytic origins, as well as components with hematological or even infectious disease origins (13, 21–47). Since some of these components are amyloid proteins (32, 45–48), the name corpora amylacea has generated certain confusion and misunderstandings over time (15). Thus, in this article, we will use the term wasteosomes (defined in ref. 15), which emphasizes the waste elements that these structures contain instead of their amyloid properties.
The wide variety of waste products described in wasteosomes has generated controversy regarding their origin or formation. Although some studies have described the presence of wasteosomes in neuronal structures (23, 49–52), evidence suggests that wasteosomes are formed in astrocytes (21, 35, 39, 42, 44, 53–57). In fact, astrocytes can capture or phagocytose residual elements and even components of synaptic boutons during neural network remodeling (58–63), which may explain the variety of components found in wasteosomes. It has been proposed that the formation of wasteosomes involves the capture of waste elements that may originate from inside or outside the cell and would involve the cell machinery required for the production of the amylopectin-like component that forms the skeleton of the container (14, 15).
Concerning the presence and abundance of wasteosomes in the brain, evidence indicates that aging is a relevant factor. Several studies indicate that wasteosomes are nonexistent in young individuals or, if present, their number is invariably low (7, 64–66), while studies that include aged individuals always report high amounts of wasteosomes at advanced ages (16, 21, 22, 29, 35, 43, 54, 67, 68). Moreover, there is a high number of studies indicating that the number of wasteosomes increases with age (13, 64, 65, 66, 69, 70). Another important factor may be sleep disorders. In this sense, a high number of wasteosomes have also been described in the brains of patients with obstructive sleep apnea in a quantitative study performed by Xu et al. (71). In that study, a large number of these structures were detected in almost all the specimens examined. The images exhibited in the article show an extraordinarily high density of wasteosomes in the brains of these patients, suggesting that the amount of wasteosomes in these cases is much higher than that observed in most other diseases. Moreover, patients with certain vascular disorders, such as small vessel disease and vascular atherosclerotic encephalopathy, also show significant amounts of wasteosomes in their brains (13, 22, 38, 68, 72). Of note, in a large series of postmortem examinations, Leel-Össy found the greatest numbers of wasteosomes in the brains of patients with vascular encephalopathies (73).
In addition to aging, sleep disorders and vascular disorders, which seem to be relevant factors associated with the formation of wasteosomes, high amounts of wasteosomes have been described in other diseases, including neurodegenerative diseases like Alzheimer’s disease (AD) (67, 74–77), Huntington’s disease (74), Parkinson’s disease (36, 77), Pick’s disease (76), amyotrophic lateral sclerosis (36), and multiple sclerosis (40, 76) as well as aging-related tau astrogliopathy (ARTAG) (72), neuromyelitis optica (44, 78), and some cases of temporal lobe epilepsy and other epilepsies (53, 79–82).
Remarkably, most of the factors that are associated with high amounts of wasteosomes are also identified as disruptive or strongly disruptive for the glymphatic system. Aging is one of the most disruptive factors for the glymphatic system (83–86), with poor sleep quality being another important disruptive factor (2, 86–91). Of note, this system shows a marked circadian rhythm and performs its cleaning function during sleep, mainly during the stages NREM 3 and NREM 2 (86). Other important strongly disruptive factors for the glymphatic system are cardiovascular disorders, which include small vessel disease (92–95), hypertension (4, 96), cerebral amyloid angiopathy (97), and vascular dementia (98).
Glymphatic disruptions have also been reported in neurodegenerative diseases (2, 86, 87, 99–102), as well as in certain types of epilepsies (103, 104), but not in neuromyelitis optica or ARTAG. However, neuromyelitis optica, in which the number of wasteosomes seems also to increase, is an astrocytopathy that occurs with the presence of antibodies directed against the AQP4 protein (44, 105, 106), which plays an essential role in the functioning of the glymphatic system. Regarding ARTAG, some alterations in the CSF flow associated with disturbances in the blood–CSF–brain barriers in basal brain regions have been described, but not specifically alterations in the glymphatic system (107). However, the disturbances of the CSF–brain barrier and the astrocytic alterations that accompany the disease (108) suggest this possibility.
It seems, therefore, that in those situations in which alterations of the glymphatic system occur, there is also an increase in wasteosomes. From our point of view, it seems conceivable that if there is a disruption of the glymphatic system, which is responsible for removing waste elements from the brain parenchyma, there will be an increase in the number of wasteosomes, as these structures accumulate waste elements. Furthermore, there is more evidence that suggests that wasteosomes are closely related to the glymphatic system.
There is a general consensus that wasteosomes are located predominantly at the perivascular, periventricular, and subpial regions of the brain. However, although the causes are unknown, they are not evenly distributed throughout these regions but are mainly found in select areas of these regions. Notably, as explained later, these select areas may correspond to the drainage regions of the glymphatic system, in which the waste elements dragged by the movement of ISF tend to concentrate, as well as to critical regions of ISF flow and critical or altered regions with an increased generation of waste products.
In 1969, Sakai et al. studied the distribution of wasteosomes in the cerebrum of four 70-y-old brains (16). The authors indicated that wasteosomes tend to concentrate in the tissue regions that are in proximity to CSF, such as the walls of the ventricles and also the brain tissue near the depths of the cerebral sulci. In addition, they produced a map of a cerebral coronal section showing the distribution and abundance of wasteosomes in different brain regions. Remarkably, the regions shown in the map as presenting the greatest numbers of wasteosomes are clearly associated with the drainage of the glymphatic system. The two regions with the highest amounts of wasteosomes are i) the medial region located at the base of the upper horns of the lateral ventricles and at the roof of the third ventricle and ii) the region located at the ventral area of the brain and close to the ambient cistern. The first region contains the internal cerebral veins (or deep cerebral veins), while the second region contains the basal vein (of Rosenthal), with all of them draining into the great cerebral vein of Galen. Remarkably, and as commented before, ISF moves, due to the glymphatic system, along an anatomical pathway toward the perivenous spaces, with a special predilection for specific perivenous spaces including that of the internal cerebral veins of the deep venous system. The third region containing numerous wasteosomes, although not at the same magnitude as that of the two regions mentioned above, is the part of the brain tissue close to the area of the lateral Sylvian sulcus. The superficial middle cerebral vein (or superficial Sylvian vein) runs through this region. Remarkably, the inferior anastomotic vein of Labbé, which contains another important perivenous space for the drainage of the glymphatic system, is a bridging vein between the Sylvian fissure and the transverse sinus (109, 110). At this point, it should be noted that the studies on the perivascular spaces which are relevant for the drainage of the glymphatic system are still incomplete and have been performed mainly in rodents. Thus, the efflux routes of ISF in the human brain are not yet known in detail. The facts presented above, however, clearly suggest a certain overlap between the presence of wasteosomes and the main drainage areas of the glymphatic system. In addition, based on the presence and location of wasteosomes, it can be predicted that the basal vein (of Rosenthal), the vein of Galen, and the Sylvian veins also play an important role in ISF drainage. In fact, it has already been suggested that the large-caliber ventral veins play an important role in the drainage of the glymphatic system (111).
The relationship between wasteosomes and the areas adjacent to the perivenous spaces is also evident if the brain regions are analyzed in more detail. In the human hippocampus, for example, wasteosomes predominate in the tissue areas close to the hippocampal sulcus and the fimbriodentate sulcus. It is precisely in these areas where the parenchymal veins of the hippocampal tissue converge toward the venous arch of the hippocampal sulcus and the venous arch of the fimbriodentate sulcus. Both venous arches drain into the basal vein, which drains into the vein of Galen (112–114). Therefore, these regions, in addition to being the regions where the majority of wasteosomes accumulate, could be important drainage areas of the glymphatic system in the hippocampus.
On the other hand, when analyzing the presence of wasteosomes in the hippocampus, we frequently observe that the fimbria of the hippocampus also contains a surprisingly high amount of wasteosomes. Although some veins cross the fimbria toward the venous arch of the fimbriodentate sulcus, the wasteosomes do not tend to localize near the fimbriodentate sulcus in this case and are distributed throughout the whole tissue. Remarkably, the fimbria is a compact white matter tract, and although white matter tracts appear to be important flow routes for ISF, the flow is highly restricted in the compact ones (2, 115). Thus, in this case, the presence of wasteosomes might be associated with the restricted flow of ISF, which can explain their distribution throughout the whole structure.
At this point, we should also highlight another region containing abundant wasteosomes that has often been overlooked in the literature: the filum terminale (116, 117). The filum terminale, consisting of glial and ependymal cells, extends from the apex of the conus medullaris to the sacrum, where it blends into the connective tissue covering this bone (117). The filum terminale has two sections. One is the filum terminale internum, which is 15 cm long and lies within the dural sac, and the other is the filum terminale externum, which is 5 cm long and lies outside the dural sac. The causes and mechanisms involved in the presence of wasteosomes in the filum terminale are unknown, but they might also be related to a deficiency in ISF flow and the consequent accumulation of waste products.
Another element that links wasteosomes to the drainage regions of the glymphatic system is the olfactory bulb. It is of note that everyone who has worked with olfactory bulb samples has observed a high presence of wasteosomes in this structure. To date, there is no reasonable explanation for this fact. However, it has been described that there is some movement of ISF from the inferior horns of the lateral ventricles toward the olfactory bulbs (118, 119) from where some of the fluid eventually drains into the nasal mucosa and its lymphatic vessels (120–123). Actually, olfactory bulbs are considered particularly relevant drainage areas of the glymphatic system (85). Once more, a high number of wasteosomes are found in a relevant drainage area of this system. Furthermore, the deficient drainage in the olfactory bulb could facilitate the entry of inhaled foreign material from the olfactory epithelium, which could in turn enhance the production of wasteosomes in the olfactory bulb. Therefore, the high presence of wasteosomes in this structure could be related to increases in both internal and external waste materials.
Thus, the select areas in which wasteosomes tend to concentrate may correspond to the drainage regions of the glymphatic system and to critical regions of ISF flow as well as critical or altered regions with an increased generation or arrival of waste products. The increase in the production of waste elements can be due, among other factors, to the presence of some stressors. In this sense, the sustained or repeated upregulation of the stress protein heme oxygenase-1 (HO-1) and the hyperinduction of glial Hmox1 by oxidative stress and other stressors accelerate the transformation of normal mitochondria into degenerative mitochondria that, engaged in a complex macroautophagic process, leads to the formation of wasteosomes (124, 125).
Apart from the possible relationship between the glymphatic system and the presence of wasteosomes, we assume in this article that the formation of wasteosomes is relatively slow or extended over time. The rarity of wasteosomes in young people and the absence of reports of acute processes involving a high number of wasteosomes support this assertion. In addition, wasteosomes are often lamellar structures, where it seems that successive layers of materials accumulate. This may be a long-lasting process since they can reach more than 50 μm in diameter. Therefore, the increase in the number of wasteosomes is likely to be related to chronic processes.
Thus, we have highlighted that the formation of wasteosomes seems to be related to the factors that cause glymphatic insufficiency, with wasteosomes localizing in the regions that are the most affected by glymphatic insufficiency. Moreover, we assume that wasteosomes are related to chronic processes. Consequently, an increase in the number of wasteosomes can be considered a hallmark of chronic glymphatic insufficiency. Assuming this, we can now comment on the repercussions of this hypothesis, which would allow us, among other things, to complete and improve the previous description of the function of wasteosomes and also to theorize about the main variables or situations that, at least chronically, have an impact on the glymphatic system.
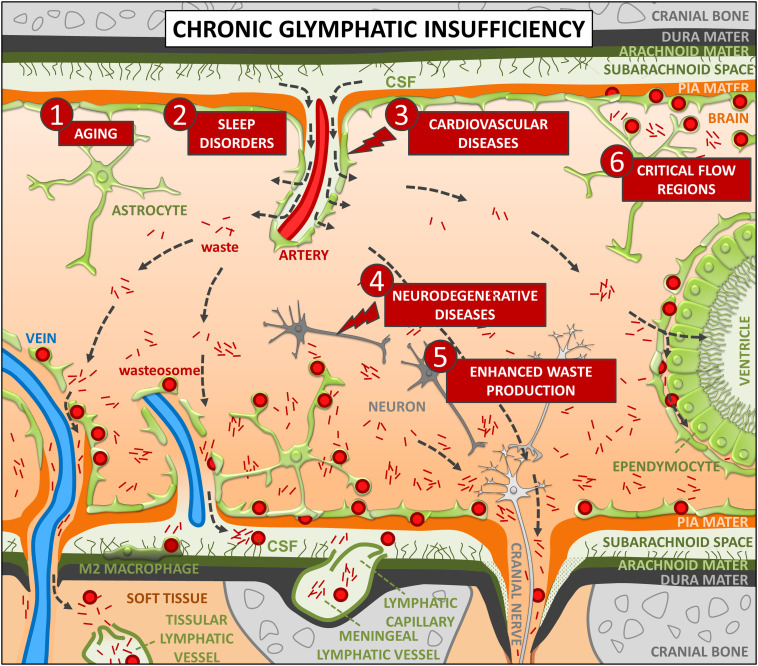
In Fig. 2, the proposed relationship between wasteosomes and the glymphatic system under chronic insufficiency is shown. Aging, sleep disorders, and cardiovascular diseases can exert a direct and chronic effect on the glymphatic system. In this case, waste elements are poorly removed and accumulate in the brain parenchyma, mainly in the drainage areas of the glymphatic system. Astrocytes phagocytose or take up some of these waste products. The waste elements that cannot be processed by the intracellular phagosome/lysosome system or the ubiquitin-proteasome system become incorporated into a resistant polyglucosan structure formed of amylopectin-like polymers, which grow over a long-lasting process, reaching more than tens of micrometers in diameter in some cases. This results in the production of wasteosomes. Hence, wasteosomes are mainly formed in the previously mentioned drainage regions of the glymphatic system, which include principally the regions near the large-caliber ventral veins and both the middle cerebral vein and the vein of Labbé, as well as the roots of the cranial and spinal nerves and the subependymal spaces. Furthermore, waste products can accumulate in specific regions of the brain parenchyma due to the restricted flow of ISF, including, for example, the fimbria of the hippocampus, the filum terminale, and the bordering subpial spaces. Wasteosomes can also accumulate in specific regions that are subjected to excessive production or arrival of waste substances, such as the olfactory bulb and some regions that present high levels of oxidative stress. In the case of neurodegenerative diseases, the formation of wasteosomes can be due to the impact of the disease on the glymphatic system, derived from the alterations of the neurovascular unit, or to increased waste production and oxidative stress generated in the affected brain areas. Regardless of the causes of wasteosome formation, the wasteosomes that are generated in the glia limitans or in proximity to CSF can be extruded by astrocytes into the CSF in an apocrine-like secretion (14). These wasteosomes can be subsequently phagocytosed by the macrophages present at central nervous system interfaces or, if entering the meningeal lymphatic vessels, by those located in the deep cervical lymph nodes. All of them are supposedly M2 macrophages and, thus, lead to protective noninflammatory responses (14, 126). Furthermore, the wasteosomes located in the most internal regions remain in the astrocytes and, therefore, accumulate in the brain. Accordingly, the number of wasteosomes in the brain tissue, although influenced by some factors like the rate of extrusion or the rate of formation of waste elements, will essentially depend on the time since the start of glymphatic insufficiency.
Fig. 2.
Hypothesized relationship between wasteosomes and the glymphatic system under chronic insufficiency. Aging (1), sleep disorders (2), and cardiovascular diseases (3) can exert a direct and chronic effect on the glymphatic system. In this case, waste elements are poorly removed and accumulate in the brain parenchyma, mainly in the drainage areas of the glymphatic system. Hence, wasteosomes are mainly formed in the regions near both the large-caliber ventral veins and some superficial veins, as well as the roots of the cranial and spinal nerves and the subependymal spaces. Neurodegenerative diseases (4) or an excessive production of waste substances in specific regions (5) can induce the local formation of wasteosomes. Due to the restricted flow of ISF, waste products can also accumulate in specific regions of the brain parenchyma like the bordering subpial spaces (6). By forming wasteosomes and incorporating waste materials into resistant polyglucosan structures formed of amylopectin-like polymers, astrocytes help in the removal and isolation of waste substances. Wasteosomes that are generated in the glia limitans or in proximity to CSF can be extruded by astrocytes into the CSF and subsequently be phagocytosed by M2 macrophages, thus leading to protective noninflammatory responses. Furthermore, wasteosomes located in the most internal regions remain in the astrocytes and, therefore, accumulate in the brain. Consequently, wasteosomes can be considered a hallmark of chronic glymphatic insufficiency. As can be seen, wasteosomes are mainly observed in perivenous, periventricular, and subpial spaces although they can also be present in some other regions. See text for details.
Analyzing the situations in which wasteosomes tend to accumulate, we can deduce those that have the greatest chronic impact on the glymphatic system. Although it has to be verified, it seems that these situations include aging, sleep disorders, and cerebrovascular pathologies, which all disturb the glymphatic system in a general way. Neurodegenerative diseases seem to have more limited effects since the correlation between these diseases and the number of wasteosomes is not systematically observed or described. However, the absence of a clear correlation might be due to possible masking effects from concomitant variables like aging, cerebrovascular pathologies, and sleep disorders. For example, in a study evaluating a broad range of pathologies in a cohort of 101 individuals, all of them presented wasteosomes, predominantly in the subpial (100%), white matter (94%), subependymal (87%), perivascular (73%), and gray matter (51%) regions (72). However, the presence of wasteosomes in the different regions did not show a significant association with the presence of Lewy body pathology, limbic-predominant age-related TDP-43 encephalopathy neuropathological change (LATE-NC), cerebral amyloid angiopathy (CAA), β-amyloid plaques, and ARTAG, except for that in the gray matter in ARTAG. Of note, the study was performed in a cohort of 101 individuals aged from 77 to 90 y, which implies that all of them probably had a high number of wasteosomes due to aging, thus possibly masking the effects of other variables. Vascular or sleep disorders, which are frequently associated with age and some of these diseases, can also mask these effects.
It can be assumed, with certain caution, that neurodegenerative diseases can trigger the formation of wasteosomes. However, the presence of wasteosomes does not necessarily imply the presence of neurodegenerative diseases, as other variables can lead to the generation of these bodies. Nevertheless, the variables that most favor chronic glymphatic insufficiency may be risk factors for the development of neurodegenerative diseases. In the case of chronic glymphatic insufficiency, although the formation of wasteosomes would help in the elimination of waste substances, the accumulation of waste substances in the parenchyma and, hence, of extracellular proteins would promote the misfolding, aggregation, and seeding of aggregation-prone proteins (86). These proteins include β-amyloid in AD; phosphorylated tau in frontotemporal dementia (FTD), chronic traumatic encephalopathy, and AD; α-synuclein in Parkinson’s disease, Lewy body disease, and multisystem atrophies; mutant huntingtin in Huntington’s disease; and TAR DNA-binding protein 43 (TDP-43) in amyotrophic lateral sclerosis and FTD (86, 127). From this point of view, it is important to know the variables that have an impact on the glymphatic system, and wasteosomes can help to ascertain them as well as their relative importance. Altogether, this knowledge could help to define the strategies and actions that can prevent or correct glymphatic disruptions, thus limiting the risk factors for developing neurodegenerative diseases.
In addition to neurodegenerative diseases, another noteworthy point is that of disorders that occur with seizures.
Wasteosome accumulation has been described in patients with temporal lobe epilepsy and other epilepsies (79, 80, 128–130). Among them, mesial temporal lobe epilepsy (MTLE) is the most common surgically remediable human epilepsy syndrome, with hippocampal sclerosis (HS) being the most frequently encountered lesion in patients with MTLE (131–133). Several reports have described the accumulation of wasteosomes in the resected hippocampus of patients with medically refractory MTLE with HS (52, 65, 130, 134–139) and its absence in MTLE with other lesions (140). Although there are still only a few studies in this regard, the presence of wasteosomes in patients with medically refractory MTLE with HS, but not in those with other lesions, could indicate that the disease progresses with chronic glymphatic insufficiency in the former. In fact, alterations in the glymphatic system in MTLE with HS have recently been described (103).
Wasteosome accumulation has also been described in local clusters that mimic low-grade glioma and result in seizures (81, 128). In one case, the patient remained seizure-free without the use of antiepileptic drugs postoperatively, suggesting that the seizures could be attributed directly to the wasteosome lesion (81). Accordingly, it is thus necessary to investigate possible glymphatic alterations associated with these lesions.
Another noteworthy case of epilepsy in this context is Lafora disease. Lafora disease is a severe, autosomal recessive progressive myoclonus epilepsy that usually manifests in previously healthy adolescents, with death commonly occurring within 10 y of symptom onset (141–143). Lafora disease is usually caused by mutations in the EPM2A gene, which encodes laforin, or by mutations in the EPM2B gene, which encodes malin, an E3 ubiquitin ligase (144–146). These proteins have been described to regulate glycogen accumulation (13, 147–149) and to intervene in the clearance of misfolded proteins (150–155). The absence of either malin or laforin results in poorly branched hyperphosphorylated glycogen that precipitates, aggregates, and accumulates into small inclusion bodies in many tissues, including the brain (13, 18, 156, 157). These inclusions, termed Lafora bodies, are the hallmark of the disease.
Studies carried out with malin knockout mice (malinKO), a mouse model of Lafora disease, show that these animals have the neuronal inclusions that are characteristic of Lafora disease and that constitute the Lafora bodies observed in the brains of patients with the condition. Moreover, these animals present a high amount of another type of cerebral inclusion that is formed in astrocytes (53). These astrocytic inclusions have been observed in many other murine models, such as SAMP8 mice (that present accelerated senescence), as well as aged C57BL/6 mice, AKR mice, and ICR-CD1 mice (158–163). Due to their granular structure and the fact that they stain with the periodic acid-Schiff (PAS) stain, these inclusions are generally called PAS granules, and since they are not exclusive to malinKO animals, they should not be considered Lafora bodies. Interestingly, these mouse PAS granules present high similarities with human wasteosomes and have been considered to be corpora amylacea-like granules (21, 53). This may indicate that there are chronic glymphatic disturbances in malinKO animals and, by extension, in Lafora disease. In this case, however, it must be taken into account that the absence of malin directly alters the metabolism of glycogen. Therefore, the presence of PAS granules could be, directly or in part, the result of this alteration in glycogen metabolism in astrocytes or perhaps the direct activation of the machinery involved in the generation of the polyglucosan structure of the waste containers (15). It should be noted, moreover, that we have not found any reports indicating the presence of abundant wasteosomes or any other type of astrocytic inclusion with a polyglucosan nature in the brains of patients with Lafora disease. We would like to highlight the reports from Cavanagh (13) and from Sakai and collaborators (156) that compared neuronal Lafora bodies with the wasteosomes from humans without this disease. This implies that the researchers knew that there are two types of bodies, but there is no reference in their studies about the presence of wasteosomes in the brains of patients with Lafora disease. Therefore, it is necessary to clarify why abundant PAS granules or astrocytic inclusions are present in malinKO animals, but wasteosomes or astrocytic inclusions are not significant or have not been described yet in patients with Lafora disease. In fact, this could perhaps help to solve why malinKO animals, despite being a model of Lafora disease, do not present the perceptible epileptic seizures typical of patients with Lafora disease.
In this article, we introduce the concept of glymphatic insufficiency and highlight that there are different elements indicating that wasteosomes are a hallmark of chronic glymphatic insufficiency. On the one hand, this premise provides an explanation that coherently links the formation, distribution, structure, and function of wasteosomes in the human brain. On the other hand, new perspectives are raised in the study of the glymphatic system since these bodies could provide information about which diseases occur with glymphatic insufficiency and which variables have the greatest impact on the glymphatic system. Almost 200 y after their discovery, corpora amylacea, mysterious and intriguing bodies, evolve to wasteosomes, bodies with a precise function related to glymphatic insufficiency. Knowing their function and their involvement in the functioning of the glymphatic system will help to understand the pathophysiology of some of the diseases of the central nervous system, helping to develop new treatments for these diseases.
Acknowledgments
This article is funded by the project I+D+i BFU2016-78398-P, from the Spanish Ministerio de Economía, Industria y Competitividad (MINECO), the Agencia Estatal de Investigación and the Fondo Europeo de Desarrollo Regional (FEDER), and funded by PID2020-115475GB-I00 / AEI / 10.13039/501100011033. We thank the Generalitat de Catalunya for funding our research group (2017/SGR625). We thank Michael Maudsley and Tasneem Ahmed for correcting the English version of the manuscript.
Author contributions
M.R., J.d.V., C.P., and J.V. designed research; M.R., J.d.V., C.P., and J.V. performed research; L.M.-P. contributed new reagents/analytic tools; M.R., J.d.V., L.M.-P., and C.P. contributed to the development of the theory; C.P. and J.V. wrote the paper and J.V. created the illustrations of the figures and developed the theory.
Competing interests
The authors declare no competing interest.
Footnotes
This article is a PNAS Direct Submission.
Data, Materials, and Software Availability
There are no data underlying this work.
References
- 1.Iliff J. J., et al. , A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci. Transl. Med. 4, 147ra111 (2012), 10.1126/scitranslmed.3003748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rasmussen M. K., Mestre H., Nedergaard M., Fluid transport in the brain. Physiol. Rev. 102, 1025–1151 (2022), 10.1152/physrev.00031.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iliff J. J., et al. , Cerebral arterial pulsation drives paravascular CSF-interstitial fluid exchange in the murine brain. J. Neurosci. 33, 18190–18199 (2013), 10.1523/JNEUROSCI.1592-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mestre H., et al. , Flow of cerebrospinal fluid is driven by arterial pulsations and is reduced in hypertension. Nat. Commun. 9, 4878 (2018), 10.1038/s41467-018-07318-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iliff J. J., et al. , Brain-wide pathway for waste clearance captured by contrast-enhanced MRI. J. Clin. Invest. 123, 1299–1309 (2013), 10.1172/JCI67677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mestre H., et al. , Aquaporin-4-dependent glymphatic solute transport in the rodent brain. Elife 7, e40070 (2018), 10.7554/eLife.40070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cserr H. F., Cooper D. N., Milhorat T. H., Flow of cerebral interstitial fluid as indicated by the removal of extracellular markers from rat caudate nucleus. Exp. Eye Res. 25, 461–473 (1977), 10.1016/s0014-4835(77)80041-9. [DOI] [PubMed] [Google Scholar]
- 8.Rennels M. L., Gregory T. F., Blaumanis O. R., Fujimoto K., Grady P. A., Evidence for a "paravascular" fluid circulation in the mammalian central nervous system, provided by the rapid distribution of tracer protein throughout the brain from the subarachnoid space. Brain Res. 326, 47–63 (1985), 10.1016/0006-8993(85)91383-6. [DOI] [PubMed] [Google Scholar]
- 9.Mestre H., Mori Y., Nedergaard M., The brain’s glymphatic system: Current controversies. Trends Neurosci. 43, 458–466 (2020), 10.1016/j.tins.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bertrand C., Diffusion and absorption within the brain: An experimental study with Prussian blue and India ink in rabbits and cats. J. Neuropathol. Exp. Neurol. 11, 53–61 (1952), 10.1097/00005072-195201000-00006. [DOI] [PubMed] [Google Scholar]
- 11.Leonhardt H., Desaga U., Recent observations on ependyma and subependymal basement membranes. Acta Neurochir. 31, 153–159 (1975), 10.1007/BF01406287. [DOI] [PubMed] [Google Scholar]
- 12.Nedergaard M., Neuroscience. Garbage truck of the brain. Science 340, 1529–1530 (2013), 10.1126/science.1240514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cavanagh J. B., Corpora-amylacea and the family of polyglucosan diseases. Brain Res. Brain Res. Rev. 29, 265–295 (1999), 10.1016/s0165-0173(99)00003-x. [DOI] [PubMed] [Google Scholar]
- 14.Riba M., et al. , Corpora amylacea act as containers that remove waste products from the brain. Proc. Natl. Acad. Sci. U.S.A. 116, 26038–26048 (2019), 10.1073/pnas.1913741116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Riba M., Del Valle J., Augé E., Vilaplana J., Pelegrí C., From corpora amylacea to wasteosomes: History and perspectives. Ageing Res. Rev. 72, 101484 (2021), 10.1016/j.arr.2021.101484. [DOI] [PubMed] [Google Scholar]
- 16.Sakai M., Austin J., Witmer F., Trueb L., Studies of corpora amylacea. I. Isolation and preliminary characterization by chemical and histochemical techniques. Arch. Neurol. 21, 526–544 (1969), 10.1001/archneur.1969.00480170098011. [DOI] [PubMed] [Google Scholar]
- 17.Sakai M., Austin J., Witmer F., Trueb L., Corpora amylacea: Isolation, characterization, and significance. Trans. Am. Neurol. Assoc. 94, 336–338 (1969). [PubMed] [Google Scholar]
- 18.Brewer M. K., et al. , Polyglucosan body structure in lafora disease. Carbohydr. Polym. 240, 116260 (2020), 10.1016/j.carbpol.2020.116260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Virchow R., Ueber eine gehirn und ruckenmark des menschen aufgefundene substanz mit der chemischen reaction der cellulose. Arch. Pathol. Anat. Physiol. Klin. Med. 6, 135–138 (1854). [Google Scholar]
- 20.Purkinje J. E., Bericht über die versammlung deutscher Naturforscher und Aerzte in Prag im september 1837. Anat. Physiol. Ver. 3, 177–180 (1838). [Google Scholar]
- 21.Augé E., Cabezón I., Pelegrí C., Vilaplana J., New perspectives on corpora amylacea in the human brain. Sci. Rep. 7, 41807 (2017), 10.1038/srep41807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Augé E., Duran J., Guinovart J. J., Pelegrí C., Vilaplana J., Exploring the elusive composition of corpora amylacea of human brain. Sci. Rep. 8, 13525 (2018), 10.1038/s41598-018-31766-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cavanagh J. B., Spinal corpora amylacea and motor neuron disease: A quantitative study. J. Neurol. Neurosurg. Psychiatry. 65, 488–491 (1998), 10.1136/jnnp.65.4.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ferraro A., Damon L. A., The histogenesis of amyloid bodies in the central nervous system. Arch. Pathol. 12, 229–244 (1931). [Google Scholar]
- 25.Hoyaux D., et al. , S100 proteins in Corpora amylacea from normal human brain. Brain Res. 867, 280–288 (2000), 10.1016/s0006-8993(00)02393-3. [DOI] [PubMed] [Google Scholar]
- 26.Iwaki T., Hamada Y., Tateishi J., Advanced glycosylation end-products and heat shock proteins accumulate in the basophilic degeneration of the myocardium and the corpora amylacea of the glia. Pathol. Int. 46, 757–763 (1996), 10.1111/j.1440-1827.1996.tb03545.x. [DOI] [PubMed] [Google Scholar]
- 27.Kawashima T., et al. , Immunohistochemical analysis in a case of idiopathic Lennox-Gastaut syndrome. Clin. Neuropathol. 18, 286–292 (1999). [PubMed] [Google Scholar]
- 28.Kerkhoff C., Klempt M., Sorg C., Novel insights into structure and function of MRP8 (S100A8) and MRP14 (S100A9). Biochim. Biophys. Acta. 1448, 200–211 (1998), 10.1016/s0167-4889(98)00144-x. [DOI] [PubMed] [Google Scholar]
- 29.Kimura T., Takamatsu J., Miyata T., Miyakawa T., Horiuchi S., Localization of identified advanced glycation end-product structures, N epsilon(carboxymethyl)lysine and pentosidine, in age-related inclusions in human brains. Pathol. Int. 48, 575–579 (1998), 10.1111/j.1440-1827.1998.tb03953.x. [DOI] [PubMed] [Google Scholar]
- 30.Li Y., et al. , S100 beta increases levels of beta-amyloid precursor protein and its encoding mRNA in rat neuronal cultures. J. Neurochem. 71, 1421–1428 (1998), 10.1046/j.1471-4159.1998.71041421.x. [DOI] [PubMed] [Google Scholar]
- 31.Libard S., et al. , Human cytomegalovirus tegument protein pp65 is detected in all intra- and extra-axial brain tumours independent of the tumour type or grade. PLoS One 9, e108861 (2014), 10.1371/journal.pone.0108861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Loeffler K. U., Edward D. P., Tso M. O., Tau-2 immunoreactivity of corpora amylacea in the human retina and optic nerve. Invest. Ophthalmol. Vis. Sci. 34, 2600–2603 (1993). [PubMed] [Google Scholar]
- 33.Meng H., Zhang X., Blaivas M., Wang M. M., Localization of blood proteins thrombospondin1 and ADAMTS13 to cerebral corpora amylacea. Neuropathology 29, 664–671 (2009), 10.1111/j.1440-1789.2009.01024.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mrak R. E., Sheng J. G., Griffin W. S., Correlation of astrocytic S100 beta expression with dystrophic neurites in amyloid plaques of Alzheimer’s disease. J. Neuropathol. Exp. Neurol. 55, 273–279 (1996), 10.1097/00005072-199603000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Navarro P. P., et al. , Cerebral Corpora amylacea are dense membranous labyrinths containing structurally preserved cell organelles. Sci. Rep. 8, 18046 (2018), 10.1038/s41598-018-36223-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pisa D., Alonso R., Rábano A., Carrasco L., Corpora amylacea of brain tissue from neurodegenerative diseases are stained with specific antifungal antibodies. Front. Neurosci. 10, 86 (2016), 10.3389/fnins.2016.00086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pisa D., Alonso R., Marina A. I., Rábano A., Carrasco L., Human and microbial proteins from corpora amylacea of Alzheimer’s disease. Sci. Rep. 8, 9880 (2018), 10.1038/s41598-018-28231-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rohn T. T., Corpora amylacea in neurodegenerative diseases: Cause or effect? Int. J. Neurol. Neurother. 2, 031 (2015), 10.23937/2378-3001/2/2/1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sbarbati A., Carner M., Colletti V., Osculati F., Extrusion of corpora amylacea from the marginal gila at the vestibular root entry zone. J. Neuropathol. Exp. Neurol. 55, 196–201 (1996), 10.1097/00005072-199602000-00008. [DOI] [PubMed] [Google Scholar]
- 40.Selmaj K., et al. , Corpora amylacea from multiple sclerosis brain tissue consists of aggregated neuronal cells. Acta Biochim. Pol. 55, 43–49 (2008). [PubMed] [Google Scholar]
- 41.Singhrao S. K., Neal J. W., Piddlesden S. J., Newman G. R., New immunocytochemical evidence for a neuronal/oligodendroglial origin for corpora amylacea. Neuropathol. Appl. Neurobiol. 20, 66–73 (1994), 10.1111/j.1365-2990.1994.tb00958.x. [DOI] [PubMed] [Google Scholar]
- 42.Schipper H. M., Cissé S., Mitochondrial constituents of corpora amylacea and autofluorescent astrocytic inclusions in senescent human brain. Glia 14, 55–64 (1995), 10.1002/glia.440140108. [DOI] [PubMed] [Google Scholar]
- 43.Steyaert A., et al. , Purification and polypeptide composition of corpora amylacea from aged human brain. J. Neurosci. Methods 31, 59–64 (1990), 10.1016/0165-0270(90)90010-d. [DOI] [PubMed] [Google Scholar]
- 44.Suzuki A., et al. , Phagocytized corpora amylacea as a histological hallmark of astrocytic injury in neuromyelitis optica. Neuropathology 32, 587–594 (2012), 10.1111/j.1440-1789.2012.01299.x. [DOI] [PubMed] [Google Scholar]
- 45.Tate-Ostroff B., Majocha R. E., Marotta C. A., Identification of cellular and extracellular sites of amyloid precursor protein extracytoplasmic domain in normal and Alzheimer disease brains. Proc. Natl. Acad. Sci. U.S.A. 86, 745–749 (1989), 10.1073/pnas.86.2.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wander C. M., et al. , The accumulation of tau-immunoreactive hippocampal granules and corpora amylacea implicates reactive glia in tau pathogenesis during aging. iScience 23, 101255 (2020), 10.1016/j.isci.2020.101255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wander C. M., et al. , Corpora amylacea are associated with tau burden and cognitive status in Alzheimer’s disease. Acta Neuropathol. Commun. 10, 110 (2022), 10.1186/s40478-022-01409-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.den Haan J., et al. , Amyloid-beta and phosphorylated tau in post-mortem Alzheimer’s disease retinas. Acta Neuropathol. Commun. 6, 147 (2018), 10.1186/s40478-018-0650-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Takahashi K., Agari M., Nakamura H., Intra-axonal corpora amylacea in ventral and lateral horns of the spinal cord. Acta Neuropathol. 31, 151–158 (1975), 10.1007/BF00688149. [DOI] [PubMed] [Google Scholar]
- 50.Mizutani T., Satoh J., Morimatsu Y., Axonal polyglucosan body in the ventral posterolateral nucleus of the human thalamus in relation to ageing. Acta Neuropathol. 74, 9–12 (1987), 10.1007/BF00688332. [DOI] [PubMed] [Google Scholar]
- 51.Woodford B., Tso M. O., An ultrastructural study of the corpora amylacea of the optic nerve head and retina. Am. J. Ophthalmol. 90, 492–502 (1980), 10.1016/s0002-9394(14)75018-4. [DOI] [PubMed] [Google Scholar]
- 52.Nishio S., et al. , Corpora amylacea replace the hippocampal pyramidal cell layer in a patient with temporal lobe epilepsy. Epilepsia 42, 960–962 (2001), 10.1046/j.1528-1157.2001.01601.x. [DOI] [PubMed] [Google Scholar]
- 53.Augé E., et al. , Astrocytes and neurons produce distinct types of polyglucosan bodies in Lafora disease. Glia 66, 2094–2107 (2018), 10.1002/glia.23463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Augé E., et al. , Corpora amylacea in human hippocampal brain tissue are intracellular bodies that exhibit a homogeneous distribution of neo-epitopes. Sci. Rep. 9, 2063 (2019), 10.1038/s41598-018-38010-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Keller J. N., Age-related neuropathology, cognitive decline, and Alzheimer’s disease. Ageing Res. Rev. 5, 1–13 (2006), 10.1016/j.arr.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 56.Palmucci L., Anzil A. P., Luh S., Intra-astrocytic glycogen granules and corpora amylacea stain positively for polyglucosans: A cytochemical contribution on the fine structural polymorphism of particulate polysaccharides. Acta Neuropathol. 57, 99–102 (1982), 10.1007/BF00685376. [DOI] [PubMed] [Google Scholar]
- 57.Ramsey H. J., Ultrastructure of corpora amylacea. J. Neuropathol. Exp. Neurol. 24, 25–39 (1965), 10.1097/00005072-196501000-00003. [DOI] [PubMed] [Google Scholar]
- 58.Chung W. S., et al. , Astrocytes mediate synapse elimination through MEGF10 and MERTK pathways. Nature 504, 394–400 (2013), 10.1038/nature12776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Konishi H., et al. , Astrocytic phagocytosis is a compensatory mechanism for microglial dysfunction. EMBO J. 39, e104464(2020), 10.15252/embj.2020104464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Konishi H., Koizumi S., Kiyama H., Phagocytic astrocytes, emerging from the shadows of microglia. Glia 70, 1009–1026 (2022), 10.1002/glia.24145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lee S. Y., Chung W. S., The roles of astrocytic phagocytosis in maintaining homeostasis of brains. J. Pharmacol. Sci. 145, 223–227 (2021), 10.1016/j.jphs.2020.12.007. [DOI] [PubMed] [Google Scholar]
- 62.Lee J. H., et al. , Astrocytes phagocytose adult hippocampal synapses for circuit homeostasis. Nature 590, 612–617 (2021), 10.1038/s41586-020-03060-3. [DOI] [PubMed] [Google Scholar]
- 63.Morizawa Y. M., et al. , Reactive astrocytes function as phagocytes after brain ischemia via ABCA1-mediated pathway. Nat. Commun. 8, 28 (2017), 10.1038/s41467-017-00037-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mrak R. E., Griffin S. T., Graham D. I., Aging-associated changes in human brain. J. Neuropathol. Exp. Neurol. 56, 1269–1275 (1997), 10.1097/00005072-199712000-00001. [DOI] [PubMed] [Google Scholar]
- 65.Chung M. H., Horoupian D. S., Corpora amylacea: A marker for mesial temporal sclerosis. J. Neuropathol. Exp. Neurol. 55, 403–408 (1996). [PubMed] [Google Scholar]
- 66.Busard H. L., et al. , Polyglucosan bodies in brain tissue: A systematic study. Clin. Neuropathol. 13, 60–63 (1994). [PubMed] [Google Scholar]
- 67.Cissé S., Perry G., Lacoste-Royal G., Cabana T., Gauvreau D., Immunochemical identification of ubiquitin and heat-shock proteins in corpora amylacea from normal aged and Alzheimer’s disease brains. Acta Neuropathol. 85, 233–240 (1993), 10.1007/BF00227716. [DOI] [PubMed] [Google Scholar]
- 68.Riba M., et al. , Corpora amylacea in the human brain exhibit neoepitopes of a carbohydrate nature. Front. Immunol. 12, 618193 (2021), 10.3389/fimmu.2021.618193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nam I. H., et al. , Association of corpora amylacea formation with astrocytes and cerebrospinal fluid in the aged human brain. Korean J. Phys. Anthropol. 25, 177–184 (2012). [Google Scholar]
- 70.Pirici D., Margaritescu C., Corpora amylacea in aging brain and age-related brain disorders. J. Aging Gerontol. 2, 33–57 (2014), 10.12974/2309-6128.2014.02.01.6. [DOI] [Google Scholar]
- 71.Xu C., Owen J. E., Gislason T., Benediktsdottir B., Robinson S. R., Quantitative analysis of size and regional distribution of corpora amylacea in the hippocampal formation of obstructive sleep apnoea patients. Sci. Rep. 11, 20892 (2021), 10.1038/s41598-021-99795-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Forrest S. L., Wagner S., Kim A., Kovacs G. G., Association of glial tau pathology and LATE-NC in the ageing brain. Neurobiol. Aging 119, 77–88 (2022), 10.1016/j.neurobiolaging.2022.07.010. [DOI] [PubMed] [Google Scholar]
- 73.Leel-Össy L., Pathological significance and characteristics of corpus amylaceum. Neuropathology 11, 105–114 (1991). [Google Scholar]
- 74.Averback P., Parasynaptic corpora amylacea in the striatum. Arch. Pathol. Lab. Med. 105, 334–335 (1981). [PubMed] [Google Scholar]
- 75.Fleming P. D., Cordoza M. E., Woods S. G., Griesbach E. J., Worcester M. A., Corpora amylacea increased in Alzheimer’s disease. Neurology 37, 57 (1987). [Google Scholar]
- 76.Singhrao S. K., Morgan B. P., Neal J. W., Newman G. R., A functional role for corpora amylacea based on evidence from complement studies. Neurodegeneration 4, 335–345 (1995), 10.1016/1055-8330(95)90024-1. [DOI] [PubMed] [Google Scholar]
- 77.Wilhelmus M. M., et al. , Novel role of transglutaminase 1 in corpora amylacea formation? Neurobiol. Aging 32, 845–856 (2011), 10.1016/j.neurobiolaging.2009.04.019. [DOI] [PubMed] [Google Scholar]
- 78.Ohara S., et al. , Neuromyelitis optica spectrum disorder with massive basal ganglia involvement: A case report. BMC Neurol. 19, 351 (2019), 10.1186/s12883-019-1580-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Radhakrishnan A., et al. , Corpora amylacea in mesial temporal lobe epilepsy: Clinico-pathological correlations. Epilepsy Res. 74, 81–90 (2007), 10.1016/j.eplepsyres.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 80.Estupiñán-Díaz B. O., et al. , Corpora amylacea in the neocortex in patients with temporal lobe epilepsy and focal cortical dysplasia. Neurologia 30, 90–96 (2015), 10.1016/j.nrl.2013.06.008. [DOI] [PubMed] [Google Scholar]
- 81.Lee S. J., et al. , Corpora amylacea mimicking low-grade glioma and manifesting as a seizure: Case report. Surg. Neurol. Int. 8, 64 (2017), 10.4103/sni.sni_423_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Leel-Össy L., Corpora L., amylacea in hippocampal sclerosis. J. Neurol. Neurosurg. Psychiatry 65, 614 (1998), 10.1136/jnnp.65.4.614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kress B. T., et al. , Impairment of paravascular clearance pathways in the aging brain. Ann. Neurol. 76, 845–861 (2014), 10.1002/ana.24271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhou Y., et al. , Impairment of the glymphatic pathway and putative meningeal lymphatic vessels in the aging human. Ann. Neurol. 87, 357–369 (2020), 10.1002/ana.25670. [DOI] [PubMed] [Google Scholar]
- 85.Jessen N. A., Munk A. S., Lundgaard I., Nedergaard M., The glymphatic system: A beginner’s guide. Neurochem. Res. 40, 2583–2599 (2015), 10.1007/s11064-015-1581-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nedergaard M., Goldman S. A., Glymphatic failure as a final common pathway to dementia. Science. 370, 50–56 (2020), 10.1126/science.abb8739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Si X., et al. , Neuroimaging evidence of glymphatic system dysfunction in possible REM sleep behavior disorder and Parkinson’s disease. NPJ Parkinsons Dis. 8, 54 (2022), 10.1038/s41531-022-00316-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yan T., Qiu Y., Yu X., Yang L., Glymphatic dysfunction: A bridge between sleep disturbance and mood disorders. Front. Psychiatry 12, 658340 (2021), 10.3389/fpsyt.2021.658340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Eide P. K., Vinje V., Pripp A. H., Mardal K. A., Ringstad G., Sleep deprivation impairs molecular clearance from the human brain. Brain 144, 863–874 (2021), 10.1093/brain/awaa443. [DOI] [PubMed] [Google Scholar]
- 90.Xie L., et al. , Sleep drives metabolite clearance from the adult brain. Science 342, 373–377 (2013), 10.1126/science.1241224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Achariyar T. M., et al. , Glymphatic distribution of CSF-derived apoE into brain is isoform specific and suppressed during sleep deprivation. Mol. Neurodegener. 11, 74 (2016), 10.1186/s13024-016-0138-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Xu J., et al. , Glymphatic dysfunction correlates with severity of small vessel disease and cognitive impairment in cerebral amyloid angiopathy. Eur. J. Neurol. 29, 2895–2904 (2022), 10.1111/ene.15450. [DOI] [PubMed] [Google Scholar]
- 93.Jiang Q., et al. , Impairment of the glymphatic system after diabetes. J. Cereb. Blood Flow Metab. 37, 1326–1337 (2017), 10.1177/0271678X16654702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mestre H., Kostrikov S., Mehta R. I., Nedergaard M., Perivascular spaces, glymphatic dysfunction, and small vessel disease. Clin. Sci. 131, 2257–2274 (2017), 10.1042/CS20160381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tian Y., Zhao M., Chen Y., Yang M., Wang Y., The underlying role of the glymphatic system and meningeal lymphatic vessels in cerebral small vessel disease. Biomolecules 12, 748 (2022), 10.3390/biom12060748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mortensen K. N., et al. , impaired glymphatic transport in spontaneously hypertensive rats. J. Neurosci. 39, 6365–6377 (2019), 10.1523/JNEUROSCI.1974-18.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chen X., et al. , Cerebral amyloid angiopathy is associated with glymphatic transport reduction and time-delayed solute drainage along the neck arteries. Nat. Aging 2, 214–223 (2022), 10.1038/s43587-022-00181-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Venkat P., et al. , White matter damage and glymphatic dysfunction in a model of vascular dementia in rats with no prior vascular pathologies. Neurobiol. Aging 50, 96–106 (2017), 10.1016/j.neurobiolaging.2016.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Rasmussen M. K., Mestre H., Nedergaard M., The glymphatic pathway in neurological disorders. Lancet Neurol. 17, 1016–1024 (2018), 10.1016/S1474-4422(18)30318-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Feng W., et al. , Microglia prevent beta-amyloid plaque formation in the early stage of an Alzheimer’s disease mouse model with suppression of glymphatic clearance. Alzheimers Res. Ther. 12, 125 (2020), 10.1186/s13195-020-00688-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zeppenfeld D. M., et al. , Association of perivascular localization of aquaporin-4 with cognition and Alzheimer disease in aging brains. JAMA Neurol. 74, 91–99 (2017), 10.1001/jamaneurol.2016.4370. [DOI] [PubMed] [Google Scholar]
- 102.Chen H. L., et al. , Associations among cognitive functions, plasma DNA, and diffusion tensor image along the perivascular space (DTI-ALPS) in patients with Parkinson’s disease. Oxid. Med. Cell Longev. 2021, 4034509 (2021), 10.1155/2021/4034509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lee D. A., et al. , Glymphatic system dysfunction in temporal lobe epilepsy patients with hippocampal sclerosis. Epilepsia Open 7, 306–314 (2022), 10.1002/epi4.12594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lee H. J., et al. , Glymphatic system dysfunction in patients with juvenile myoclonic epilepsy. J. Neurol. 269, 2133–2139 (2022), 10.1007/s00415-021-10799-w. [DOI] [PubMed] [Google Scholar]
- 105.Lennon V. A., Kryzer T. J., Pittock S. J., Verkman A. S., Hinson S. R., IgG marker of optic-spinal multiple sclerosis binds to the aquaporin-4 water channel. J. Exp. Med. 202, 473–477 (2005), 10.1084/jem.20050304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Roemer S. F., Pattern-specific loss of aquaporin-4 immunoreactivity distinguishes neuromyelitis optica from multiple sclerosis. Brain 130, 1194–1205 (2007), 10.1093/brain/awl371. [DOI] [PubMed] [Google Scholar]
- 107.Kovacs G. G., et al. , Sequential stages and distribution patterns of aging-related tau astrogliopathy (ARTAG) in the human brain. Acta Neuropathol. Commun. 6, 50 (2018), 10.1186/s40478-018-0552-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kovacs G. G., Yousef A., kaindl S., Lee V. M., Trojanowski J. Q., Connexin-43 and aquaporin-4 are markers of ageing-related tau astrogliopathy (ARTAG)-related astroglial response. Neuropathol. Appl. Neurobiol. 44, 491–505 (2018), 10.1111/nan.12427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mincă D. I., Rusu M. C., Rădoi P. M., Hostiuc S., Toader C., A new classification of the anatomical variations of Labbé’s inferior anastomotic vein. Tomography 8, 2182–2192 (2022), 10.3390/tomography8050183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Boukobza M., Crassard I., Bousser M. G., Chabriat H., Labbé vein thrombosis. Neuroradiology 62, 935–945 (2020), 10.1007/s00234-020-02396-x. [DOI] [PubMed] [Google Scholar]
- 111.Louveau A., et al. , Understanding the functions and relationships of the glymphatic system and meningeal lymphatics. J. Clin. Invest. 127, 3210–3219 (2017), 10.1172/JCI90603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Tatu L., Vuillier F., Structure and vascularization of the human hippocampus. Front. Neurol. Neurosci. 34, 18–25 (2014), 10.1159/000356440. [DOI] [PubMed] [Google Scholar]
- 113.Duvernoy H., The Superficial Veins of the Human Brain (Springer, Berlin, 1975). [Google Scholar]
- 114.Huang Y., Wolf B., "The basal cerebral vein and its tributaries" in Radiology of the Skull and Brain, Newton T., Potts D., Eds. (Mosby, St. Louis, 1974), pp. 2111–2154. [Google Scholar]
- 115.Wang A., et al. , The drainage of interstitial fluid in the deep brain is controlled by the integrity of myelination. Aging Dis. 10, 937–948 (2019), 10.14336/AD.2018.1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Harmeier J., The normal histology of the intradural filum terminale. Arch. Neurol. Psychiatry 29, 308–316 (1933). [Google Scholar]
- 117.Saker E., Wilson C., Tubbs R. S., "The filum terminale" in Occult Spinal Dysraphism, Tubbs R., Oskouian R., Blount J., Oakes W., Eds. (Springer, Cham, 2019), 10.1007/978-3-030-10994-3_5. [DOI] [Google Scholar]
- 118.Clarke J., Cell migration: Neurons go with the flow. Curr. Biol. 16, R337–R339 (2006), 10.1016/j.cub.2006.03.079. [DOI] [PubMed] [Google Scholar]
- 119.Curtis M. A., et al. , Human neuroblasts migrate to the olfactory bulb via a lateral ventricular extension. Science. 315, 1243–1249 (2007), 10.1126/science.1136281. [DOI] [PubMed] [Google Scholar]
- 120.Johnston M., Zakharov A., Papaiconomou C., Salmasi G., Armstrong D., Evidence of connections between cerebrospinal fluid and nasal lymphatic vessels in humans, non-human primates and other mammalian species. Cerebrospinal Fluid Res. 1, 2 (2004), 10.1186/1743-8454-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Koh L., Zakharov A., Johnston M., Integration of the subarachnoid space and lymphatics: Is it time to embrace a new concept of cerebrospinal fluid absorption? Cerebrospinal Fluid Res. 2, 6 (2005), 10.1186/1743-8454-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Murtha L. A., et al. , Cerebrospinal fluid is drained primarily via the spinal canal and olfactory route in young and aged spontaneously hypertensive rats. Fluids Barriers CNS 11, 12 (2014), 10.1186/2045-8118-11-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Praetorius J., Water and solute secretion by the choroid plexus. Pflugers Arch. 454, 1–18 (2007), 10.1007/s00424-006-0170-6. [DOI] [PubMed] [Google Scholar]
- 124.Schipper H. M., Brain iron deposition and the free radical-mitochondrial theory of ageing. Ageing Res. Rev. 3, 265–301 (2004), 10.1016/j.arr.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 125.Schipper H. M., Song W., Tavitian A., Cressatti M., The sinister face of heme oxygenase-1 in brain aging and disease. Prog. Neurobiol. 172, 40–70 (2019), 10.1016/j.pneurobio.2018.06.008. [DOI] [PubMed] [Google Scholar]
- 126.Riba M., Wasteosomes (corpora amylacea) of human brain can be phagocytosed and digested by macrophages. Cell Biosci. 12, 177 (2022). 10.1186/s13578-022-00915-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Soto C., Pritzkow S., Protein misfolding, aggregation, and conformational strains in neurodegenerative diseases. Nat. Neurosci. 21, 1332–1340 (2018), 10.1038/s41593-018-0235-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Abel T. J., Hebb A. O., Keene C. D., Born D. E., Silbergeld D. L., Parahippocampal corpora amylacea: Case report. Neurosurgery 66, E1206–E1207 (2010), 10.1227/01.NEU.0000369196.94664.4E. [DOI] [PubMed] [Google Scholar]
- 129.Das A., et al. , Corpora amylacea deposition in the hippocampus of patients with mesial temporal lobe epilepsy: A new role for an old gene? Indian J. Hum. Genet. 17, S41–S47 (2011), 10.4103/0971-6866.80358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kawamura T., Morioka T., Nishio S., Fukui K., Fukui M., Temporal lobe epilepsy and corpora amylacea in the hippocampus: Clinicopathologic correlation. Neurol. Res. 24, 563–569. [DOI] [PubMed] [Google Scholar]
- 131.Engel J. Jr., et al. , Practice parameter: Temporal lobe and localized neocortical resections for epilepsy: Report of the quality standards subcommittee of the American academy of neurology, in association with the American epilepsy society and the American association of neurological surgeons. Neurology 60, 538–547 (2003), 10.1212/01.wnl.0000055086.35806.2d. [DOI] [PubMed] [Google Scholar]
- 132.McIntosh A. M., Wilson S. J., Berkovic S. F., Seizure outcome after temporal lobectomy: Current research practice and findings. Epilepsia 42, 1288–1307 (2001), 10.1046/j.1528-1157.2001.02001.x. [DOI] [PubMed] [Google Scholar]
- 133.Wieser H. G., ILAE commission on neurosurgery of epilepsy. ILAE commission report. Mesial temporal lobe epilepsy with hippocampal sclerosis. Epilepsia 45, 695–714 (2004), 10.1111/j.0013-9580.2004.09004.x. [DOI] [PubMed] [Google Scholar]
- 134.Cherian P. J., Radhakrishnan V. V., Radhakrishnan K., The significance of corpora amylacea in mesial temporal lobe epilepsy. Neurol. India 51, 277–279 (2003). [PubMed] [Google Scholar]
- 135.Loiseau H., et al. , Occurrence of polyglucosan bodies in temporal lobe epilepsy. J. Neurol. Neurosurg. Psychiatry 55, 1092–1093 (1992), 10.1136/jnnp.55.11.1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.MacKenzie J. M., Polyglucosan bodies are not an unusual finding in temporal lobe epilepsy. J. Neurol. Neurosurg. Psychiatry 56, 577 (1993), 10.1136/jnnp.56.5.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ribeiro M. d. C., et al. , Corpora amylacea in temporal lobe epilepsy associated with hippocampal sclerosis. Arq. Neuropsiquiatr. 61, 942–945 (2003), 10.1590/s0004-282x2003000600010. [DOI] [PubMed] [Google Scholar]
- 138.Streichenberger N., Polyglucosan bodies and temporal lobe epilepsy: An incidental finding or more? Clin. Neuropathol. 20, 172–175 (2001). [PubMed] [Google Scholar]
- 139.Van Paesschen W., Revesz T., Duncan J. S., Corpora amylacea in hippocampal sclerosis. J. Neurol. Neurosurg. Psychiatry 63, 513–515 (1997), 10.1136/jnnp.63.4.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Radhakrishnan V. V., et al. , Pathology of temporal lobe epilepsy: An analysis of 100 consecutive surgical specimens from patients with medically refractory epilepsy. Neurol. India 47, 196–201 (1999). [PubMed] [Google Scholar]
- 141.Delgado-Escueta A. V., Ganesh S., Yamakawa K., Advances in the genetics of progressive myoclonus epilepsy. Am. J. Med. Genet. 106, 129–138 (2001), 10.1002/ajmg.1575. [DOI] [PubMed] [Google Scholar]
- 142.Girard J. M., Turnbull J., Ramachandran N., Minassian B. A., Progressive myoclonus epilepsy. Handb. Clin. Neurol. 113, 1731–1736 (2013), 10.1016/B978-0-444-59565-2.00043-5. [DOI] [PubMed] [Google Scholar]
- 143.Nitschke F., Ahonen S. J., Nitschke S., Mitra S., Minassian B. A., Lafora disease - from pathogenesis to treatment strategies. Nat. Rev. Neurol. 14, 606–617 (2018), 10.1038/s41582-018-0057-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Chan E. M., et al. , Mutations in NHLRC1 cause progressive myoclonus epilepsy. Nat. Genet. 35, 125–127 (2003), 10.1038/ng1238. [DOI] [PubMed] [Google Scholar]
- 145.Minassian B. A., et al. , Mutations in a gene encoding a novel protein tyrosine phosphatase cause progressive myoclonus epilepsy. Nat. Genet. 20, 171–174 (1998), 10.1038/2470. [DOI] [PubMed] [Google Scholar]
- 146.Serratosa J. M., et al. , A novel protein tyrosine phosphatase gene is mutated in progressive myoclonus epilepsy of the Lafora type (EPM2). Hum. Mol. Genet. 8, 345–352 (1999), 10.1093/hmg/8.2.345. [DOI] [PubMed] [Google Scholar]
- 147.Cheng A., et al. , A role for AGL ubiquitination in the glycogen storage disorders of Lafora and Cori’s disease. Genes Dev. 21, 2399–2409 (2007), 10.1101/gad.1553207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Vilchez D., et al. , Mechanism suppressing glycogen synthesis in neurons and its demise in progressive myoclonus epilepsy. Nat. Neurosci. 10, 1407–1413 (2007), 10.1038/nn1998. [DOI] [PubMed] [Google Scholar]
- 149.Worby C. A., Gentry M. S., Dixon J. E., Malin decreases glycogen accumulation by promoting the degradation of protein targeting to glycogen (PTG). J. Biol. Chem. 283, 4069–4076 (2008), 10.1074/jbc.M708712200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Aguado C., et al. , Laforin, the most common protein mutated in Lafora disease, regulates autophagy. Hum. Mol. Genet. 19, 2867–2876 (2010), 10.1093/hmg/ddq190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Criado O., et al. , Lafora bodies and neurological defects in malin-deficient mice correlate with impaired autophagy. Hum. Mol. Genet. 21, 1521–1533 (2012), 10.1093/hmg/ddr590. [DOI] [PubMed] [Google Scholar]
- 152.Garyali P., et al. , The malin-laforin complex suppresses the cellular toxicity of misfolded proteins by promoting their degradation through the ubiquitin-proteasome system. Hum. Mol. Genet. 18, 688–700 (2009), 10.1093/hmg/ddn398. [DOI] [PubMed] [Google Scholar]
- 153.Knecht E., et al. , Impaired autophagy in Lafora disease. Autophagy 6, 991–993 (2010), 10.4161/auto6.7.13308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Puri R., Suzuki T., Yamakawa K., Ganesh S., Dysfunctions in endosomal-lysosomal and autophagy pathways underlie neuropathology in a mouse model for Lafora disease. Hum. Mol. Genet. 21, 175–184 (2012), 10.1093/hmg/ddr452. [DOI] [PubMed] [Google Scholar]
- 155.Rao S. N., et al. , Sequestration of chaperones and proteasome into Lafora bodies and proteasomal dysfunction induced by Lafora disease-associated mutations of malin. Hum. Mol. Genet. 19, 4726–4734 (2010), 10.1093/hmg/ddq407. [DOI] [PubMed] [Google Scholar]
- 156.Sakai M., Austin J., Witmer F., Trueb L., Studies in myoclonus epilepsy (Lafora body form): II. Polyglucosans in the systemic deposits of myoclonus epilepsy and in corpora amylacea. Neurology 20, 160–176 (1970), 10.1212/wnl.20.2.160. [DOI] [PubMed] [Google Scholar]
- 157.Nitschke F., et al. , Abnormal glycogen chain length pattern, not hyperphosphorylation, is critical in Lafora disease. EMBO Mol. Med. 9, 906–917 (2017), 10.15252/emmm.201707608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Akiyama H., et al. , Periodic acid-Schiff (PAS)-positive, granular structures increase in the brain of senescence accelerated mouse (SAM). Acta Neuropathol. 72, 124–129 (1986), 10.1007/BF00685973. [DOI] [PubMed] [Google Scholar]
- 159.Jucker M., et al. , Age-associated inclusions in normal and transgenic mouse brain. Science 255, 1443–1445 (1992), 10.1126/science.1542796. [DOI] [PubMed] [Google Scholar]
- 160.Lamar C. H., Hinsman E. J., Henrikson C. K., Alterations in the hippocampus of aged mice. Acta Neuropathol. 36, 387–391 (1976), 10.1007/BF00699644. [DOI] [PubMed] [Google Scholar]
- 161.Manich G., et al. , Clustered granules present in the hippocampus of aged mice result from a degenerative process affecting astrocytes and their surrounding neuropil. Age 36, 9690 (2014), 10.1007/s11357-014-9690-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Manich G., Cabezón I., Augé E., Pelegrí C., Vilaplana J., Periodic acid-Schiff granules in the brain of aged mice: From amyloid aggregates to degenerative structures containing neo-epitopes. Ageing Res. Rev. 27, 42–55 (2016), 10.1016/j.arr.2016.03.001. [DOI] [PubMed] [Google Scholar]
- 163.Mitsuno S., et al. , Immunohistochemical, conventional and immunoelectron microscopical characteristics of periodic acid-Schiff-positive granules in the mouse brain. Acta Neuropathol. 98, 31–38 (1999), 10.1007/s004010051048. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
There are no data underlying this work.