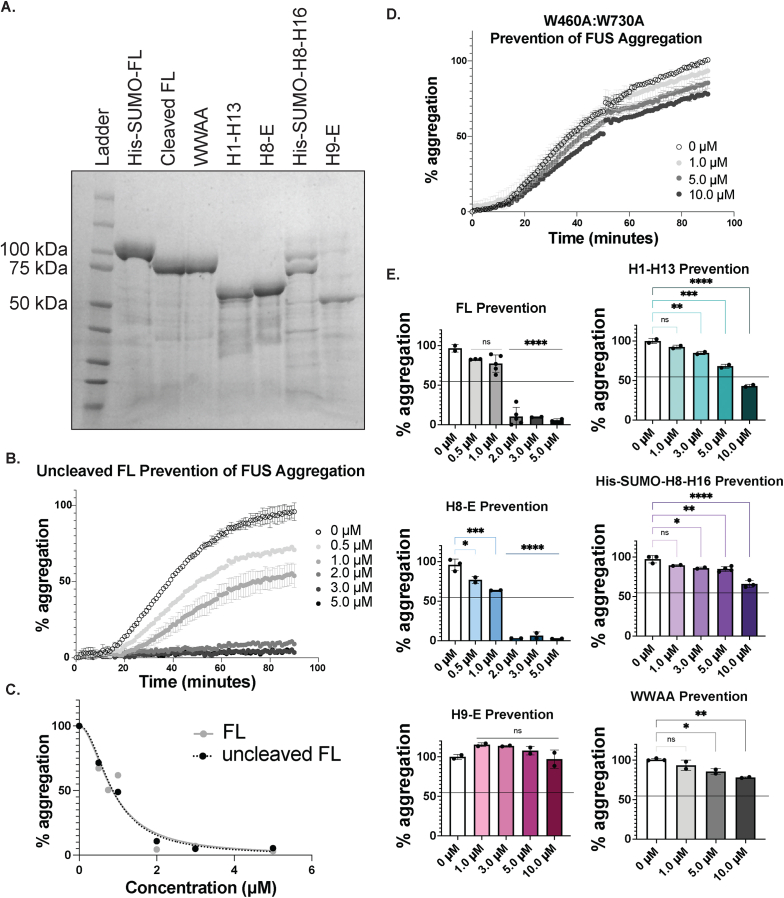
Supplemental Figure S2.
Kapβ2 and H8-E robustly suppress FUS fibrillization.A, 5 μg of purified His-SUMO-FL Kapβ2, cleaved FL Kapβ2, H1-H13, H8-E, His-SUMO-H8-H16, and H9-E were processed for SDS-PAGE and stained with Coomassie. B, to measure the ability of His-SUMO-tagged Kapβ2 to prevent FUS fibrillization, we incubated 3 μM FUS with 1 μg TEV protease and the indicated concentration of His-SUMO-tagged Kapβ2 and monitored turbidity at 395 nm. Increased turbidity indicates FUS fibrillization and each curve represents the mean of 2 trials ± SD. For each experiment, the maximal value of FUS turbidity in the absence of Kapβ2 was set to 100 and all data for that trial were normalized to that value. C, to compare uncleaved and cleaved FL Kapβ2 chaperone activity, we calculated the area under the curve (AUC) for each concentration and set the AUC for FUS alone as 100 to normalize all other conditions to produce percent aggregation. Overlaying the plot of concentration versus aggregation (%) for cleaved and uncleaved FL Kapβ2 shows that the uncleaved protein works as well as cleaved Kapβ2 in preventing the fibrillization of FUS, suggesting that the His-SUMO tag itself neither hinders nor enhances Kapβ2 chaperone activity in this assay, although it is likely stabilizing H8-H16. D, we assessed the ability of Kapβ2W460A:W730A (WWAA) to chaperone FUS. We incubated 3 μM FUS with 1 μg TEV protease and the indicated concentration of Kapβ2W460A:W730A and monitored turbidity at 395 nm. Each curve represents the mean of 2 to 3 trials ± SD. The maximal value of FUS turbidity in the absence of Kapβ2 was set to 100 and all data for that trial were normalized to that value. E, in Figure 4, we assessed the ability of FL and truncated Kapβ2 constructs to chaperone FUS. We incubated 3 μM FUS with 1 μg TEV protease and the indicated concentration of each Kapβ2 construct and monitored turbidity at 395 nm for 90 min. Here, only the final normalized absorbance reading (where the maximum value for FUS alone is set as 100) is plotted for each Kapβ2 construct at each of the indicated concentrations. Data points represent individual trials and bars represent means ± SD. An ordinary one-way ANOVA with Dunnett’s multiple comparisons test was used to compare the final time point of each condition to FUS alone for that construct. ∗p < 0.03; ∗∗p < 0.008; ∗∗∗p < 0.0003; ∗∗∗∗p < 0.0001.