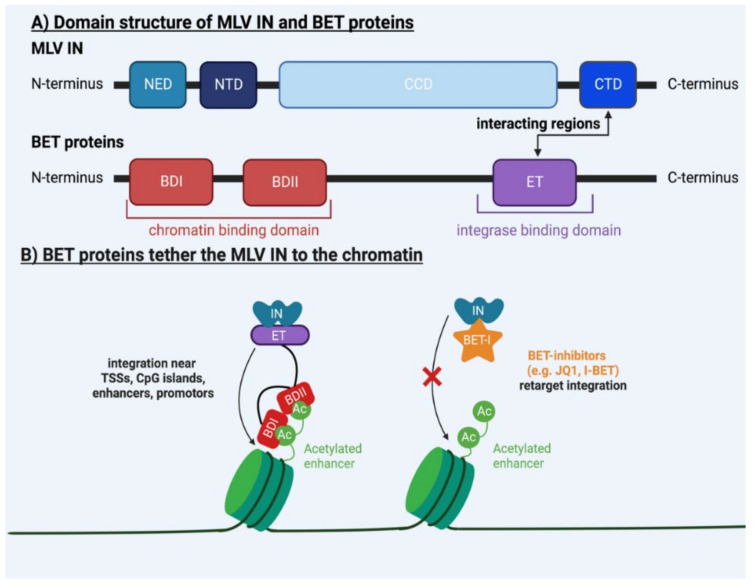
Figure 1.
(A) Domain structure of murine leukemia virus (MLV) integrase (IN) and bromodomain and extra–terminal motif (BET) proteins. The MLV IN consists of an N–terminal extension domain (NED), an N–terminal domain (NTD), a catalytic core domain (CCD) and a C–terminal domain (CTD) [4]. BET proteins consist of two bromodomains (BD), BDI and BDII, that bind acetylated lysines in the nucleosomes, and an extra–terminal (ET) domain that interacts with the CTD of the MLV IN [28,29,30,31,32,33,34]. (B) BET proteins tether the MLV IN. Through the combined interaction of the ET domain of BET proteins (which binds the CTD of the MLV IN) and BDI and BDII (which bind the acetylated chromatin), BET proteins tether the MLV IN to acetylated chromatin regions such as enhancers [28,29,30,31,32,33,34]. BET inhibitors, such as JQ1 and I–BET, retarget the integration of MLV away from BET–recognized sites by uncoupling the interaction between BET proteins and MLV IN [29,30,31]. (Figure created with Biorender.com on 17 December 2022).