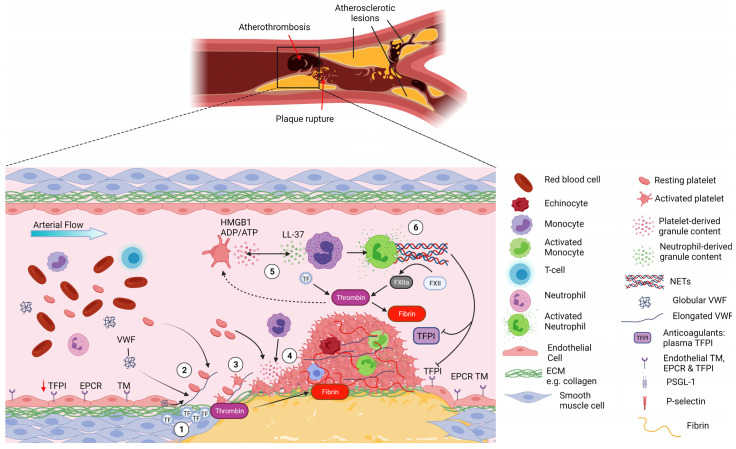
Figure 2.
Platelet–neutrophil interactions in atherothrombosis. Atherothrombosis is characterised by atherosclerotic plaque rupture or erosion, exposing collagen-rich plaque material to the blood and causing subsequent thrombus formation. 1- Upon plaque rupture the subendothelial matrix becomes exposed, allowing VWF to bind collagen and exposing TF, initiating the extrinsic coagulation pathway. 2- Upon the shear forces of the blood, VWF unravels and binds platelets via GPIbα. The fast on and off rates of the VWF-GPIbα interaction allow the deceleration of platelets and their binding to collagen receptors. 3- Platelets become activated and start forming aggregates while releasing their granule content. 4- This promotes the recruitment of additional platelets but also neutrophils to the site of vascular injury via HMGB1 binding to neutrophil RAGE receptor. 5- Once recruited, neutrophils become activated and release their granule content which further activates platelets (e.g., LL-37 activating GPVI platelet receptor). Blood born TF from monocyte microvesicles further augments the extrinsic coagulation pathway leading to thrombin release in the milieu. 6- Progressively, the thrombus grows, mostly composed of platelets and fibrin that consolidate the thrombus but with the presence of neutrophils. Platelet-induced activation of neutrophils triggers the formation of NETs that further augment the coagulation system via activation of the contact pathway and inhibiting anticoagulant TFPI. Ultimately, the overwhelming activation of the coagulation pathways and platelets lead to excessive thrombus formation and arterial occlusion in myocardial infraction and stroke. Created by Biorender.com.