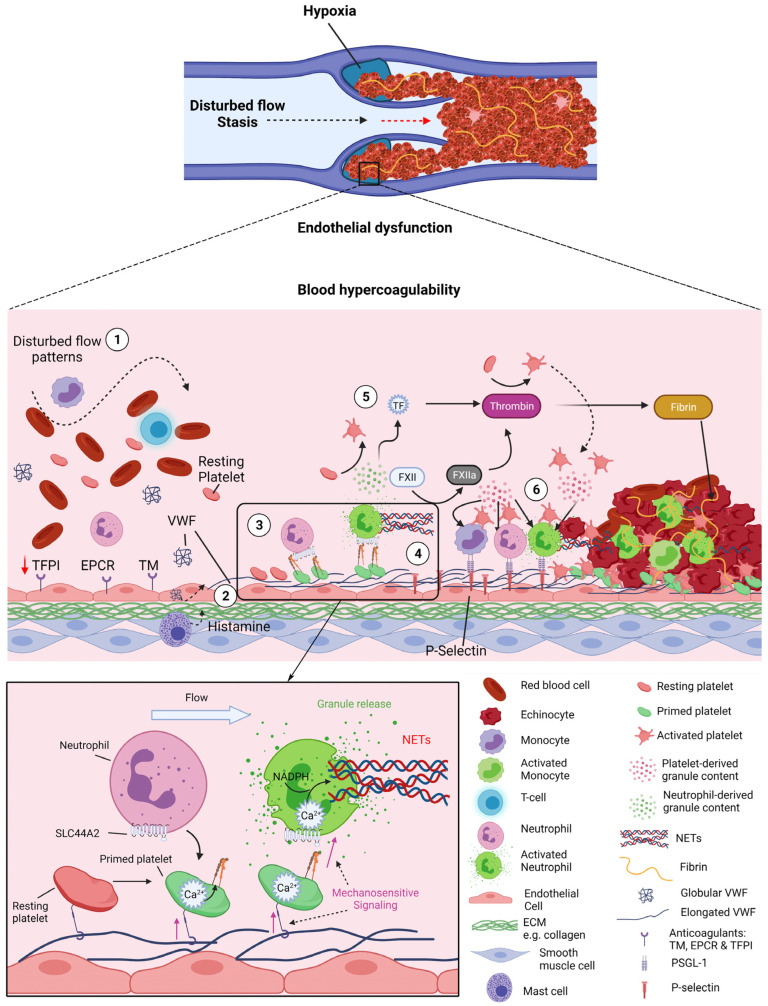
Figure 3.
Platelet–neutrophil crosstalk in venous thrombosis. Venous thrombosis initiates in the pockets of venous valves where disturbed flow creates a prothrombotic environment: hypoxia, endothelial dysfunction and blood hypercoagulability. 1- These flow patterns/stasis induces downregulation of antithrombotic factors of the endothelium thrombomodulin (TM), endothelial protein C receptor (EPCR) and tissue factor pathway inhibitor (TFPI) and concomitant upregulation of procoagulant proteins von Willebrand Factor (VWF), P-selectin and other cell adhesion molecules. Mast cells are also thought to be involved in this process by releasing histamine. 2- Under these flow disturbance and pro-coagulant conditions, VWF can become tangled and unravel, exposing its A1 domain enabling platelet binding via GPIbα. 3- The VWF-GPIbα interaction under flow induces the mechano-unfolding of the mechanosensitive domain of GPIbα leading to signalling events, Ca2+ release from intracellular stores and activation of αIIbβ3. 4- Primed platelets via activated αIIbβ3 can bind SLC44A2 on neutrophils under flow. Shear forces in the neutrophil induce calcium- and NADPH-mediated NET formation. 5- Activated neutrophils can directly activate platelets through release of granules (e.g., LL-37) or indirectly by generating thrombin via NETs. NETs can directly activate the intrinsic coagulation pathway by binding to FXII and inhibit the anticoagulant protein TFPI, augmenting thrombin generation. Granule content from both platelets and neutrophils further stimulates the endothelium leading to increased expression of P-selectin and other adhesion molecules. 6- Monocytes and neutrophils are recruited to the endothelium via PSGL-1 binding to endothelial P-selectin. Activated platelets are able to bind activated neutrophils and monocytes and further stimulate them by release of their content (e.g., HMGB1, cytokines). NETs promote thrombus development by binding red blood cells, VWF and platelets, activating of both intrinsic and extrinsic coagulation pathways, and also facilitate thrombus stability by providing fibrinolytic resistance. Ultimately venous thrombi rich in red blood cells and fibrin obstruct the valve and upstream vein, causing DVT. Created by Biorender.com.