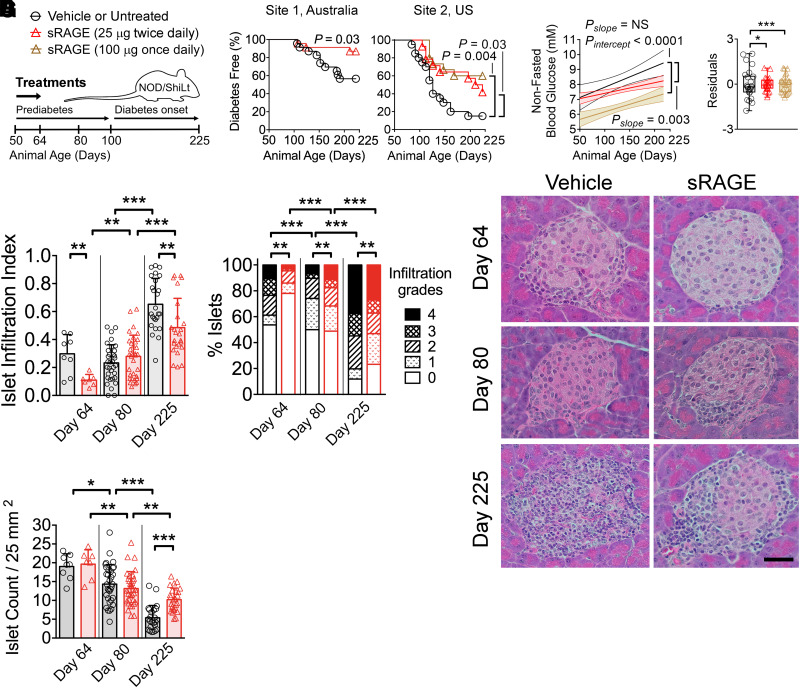
Figure 1.
Treatment with sRAGE provides lasting protection against autoimmune diabetes in an international multisite preclinical trial. A: NOD/ShiLt mice were administered vehicle at site 1 or were untreated at site 2 (black bars/circles) or treated with 25 µg sRAGE twice daily (red bars/triangles) or 100 µg sRAGE once daily (brown bars/triangles) from days 50–64 of life. B: Autoimmune diabetes incidence. Site 1, three independent experiments, n = 23/group; site 2, one independent experiment, n = 14–20/group. C: Nonfasting blood glucose concentrations shown as linear regression ± 95% CI (left) and residuals representing variability of blood glucose levels from the regression line (right). D–F: Pancreatic islet infiltration. D: Islet infiltration index (0 indicates no infiltration; to 1 indicates >75% infiltration). E: Degree of islet infiltration (grade 0, none; grade 1, peri-infiltration; grade 2, <25% infiltration; grade 3, 25–75% infiltration; grade 4, >75% infiltration). F: Representative hematoxylin-eosin photomicrographs (n = 7–33 sections/group from n = 4–7 mice/group; scale bar = 40 µm). G: Islet count normalized by tissue area. Column graphs are shown as median (IQR), with analysis with two-tailed Mann-Whitney U test. Box-and-whisker plot variances were analyzed with F test. Degree of insulitis is shown as mean and proportions analyzed with Fischer exact test. Diabetes incidence is shown with Kaplan-Meier survival curves and was analyzed with log-rank test. *P < 0.05; **P < 0.01; ***P < 0.001.