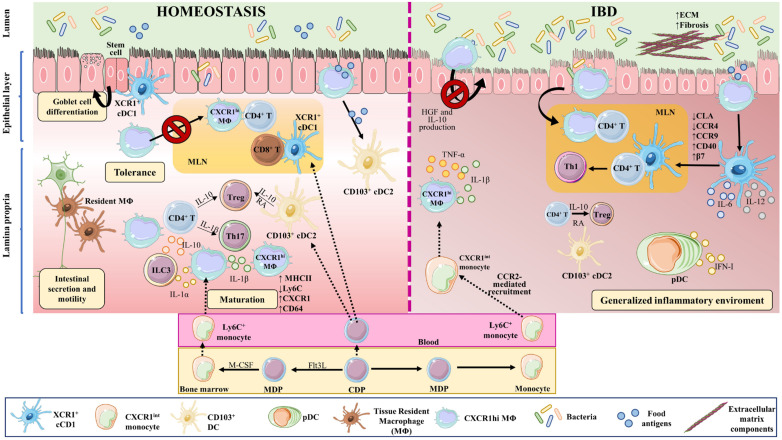
Figure 2.
Macrophages and dendritic cell in homeostasis and IBD. Macrophages and DCs play important roles in homeostasis and in the development of IBD by phagocytosing cellular debris, producing cytokines, regulating tissue repair, and interacting with other cells. In the intestine, monocyte-derived macrophages (MΦ) are more abundant than tissue-resident macrophages of embryonic origin. Both perform phagocytosis, produce cytokines, and interact with other cells. Upon weaning, bone marrow-derived monocytes egress from the circulation and extravasate into the tissue, where they undergo differentiation and maturation (downregulation of Ly6C, production of MHCII, and increased expression of CX3CR1). In homeostasis, tissue-resident macrophages in the muscularis externa interact with enteric and myenteric neurons controlling intestinal secretion and motility, while in the lamina propria, macrophages provide signals to intestinal stem cells that give rise to goblet cells, Paneth cells, and intestinal epithelial cells. These macrophages also modulate T cell activities and functions, via the secretion of IL-10 for Tregs and IL-1β for Th17 cells. In addition, they affect ILC3 cells through the production of IL-1α and IL-1β. The migration of antigen-loaded CX3CR1high intestinal macrophages to mesenteric lymph nodes is impaired by intestinal microbiota, thus affecting antigen presentation to T cells and effectively sustaining tolerance towards commensal bacteria. On the other hand, XCR1+ DCs play a tolerogenic role upon recognizing commensal bacterial components, while CD103+ cDC2s seem to be important for initiating oral tolerance through their capacity to generate RA and IL-10. In IBD, large numbers of Ly6Chigh inflammatory monocytes are recruited to the intestine in a CCR2-dependent manner, becoming pro-inflammatory effector cells. These inflammatory macrophages produce TNFα, IL-6, and iNOS, and directly cause the onset and development of fibrosis through a disproportionate accumulation of ECM. The intestinal microbiota is impaired during chronic colitis, and CX3CR1high macrophages can change their habits and migrate to lymph nodes. CD103+ cDC numbers are significantly reduced in the inflamed and uninflamed intestine in IBD; however, activated DCs can release inflammatory cytokines, in addition to type I IFN produced by pDCs. All these phenomena contribute to a generalized inflammation. Part of the figure was generated by using pictures from Servier Medical Art.