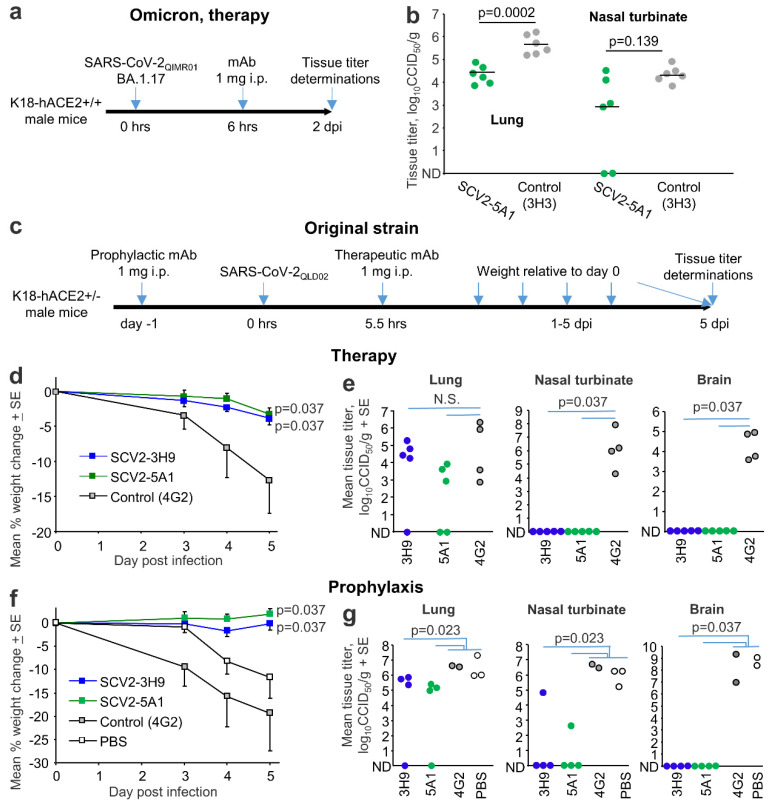
Figure 6.
Protection in K18-ACE2 mice. (a) Timeline of mAb therapy of K18-ACE2+/+ mice infected with a BA.1 omicron isolate (SARS-CoV-2QIMR01). The anti-SARS-CoV-2 antibody was SCV2-5A1 and the control mAb was an isotype control, anti-Zika virus NS1 (3H3). (b) Lung and nasal turbinate titers for mice described in a. Statistics by t test (lung) and Kolmogorov–Smirnov test (nasal turbinates). (c) Timelines for prophylactic and therapeutic treatment of K18-ACE2+/− mice infected with an original strain isolate (SARS-CoV-2QLD02) with SCV2-5A1 or SCV2-3H9, or the control mAb, anti-flavivirus E (4G2). (d) Mean weight change relative to 0 dpi after mAb therapy. Statistics by Kolmogorov–Smirnov tests for 5 dpi. (e) Tissue titrations for the same mice shown in d (5 dpi). Statistics by Kolmogorov–Smirnov tests. N.S.—not significant. (f) Mean weight change relative to 0 dpi after mAb prophylaxis. For statistics, data for 4G2 and PBS were combined to represent the controls (n = 5). Statistics by Kolmogorov–Smirnov tests on 5 dpi. (g) Tissue titrations for the same mice are shown in f (5 dpi). Statistics by Kolmogorov–Smirnov tests, with 4G2 and PBS groups combined.