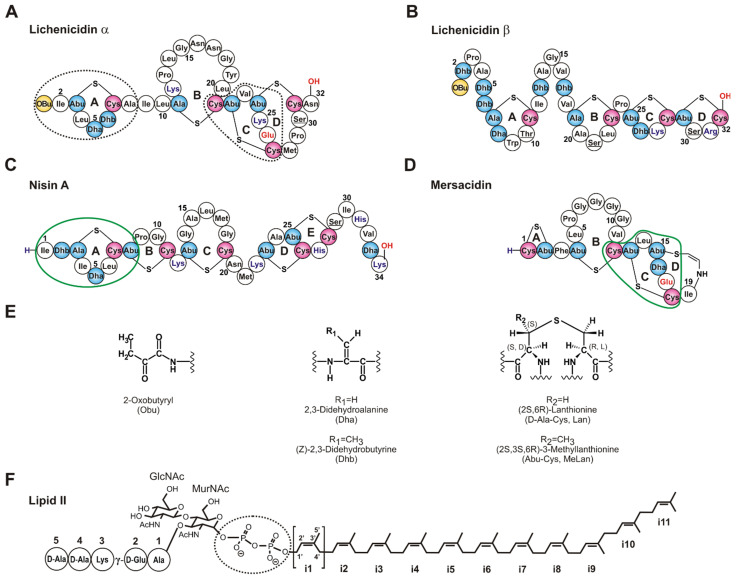
Figure 1.
Structures of some of the lantibiotics mentioned in this work and lipid II. (A,B) The α- and β-components of lichenicidin (Lchα and Lchβ) [18]. It is worth noting that the arrangement of the ring A in Lchα differs from that in lichenicidin I89 [19]. The residues that are derived from Ser/Thr are shown in blue (Dha/Dhb/Ala/Abu), red (Cys), and yellow (Obu). Unmodified Ser/Thr are underlined. (C,D) Nisin A and mersacidin—classic examples of type A and type B lantibiotics containing different lipid II binding sites. (E) The chemical structures of post-translationally modified amino acid residues. (F) The structure of lipid II with Lys residue in the 3rd position of the pentapeptide. Isoprene repeats of the bactoprenol moiety are sequentially labeled i1–i11. The pyrophosphate group in lipid II, the lipid II-binding sites in nisin and mersacidin, and the putative lipid II-binding sites in Lchα are encircled.