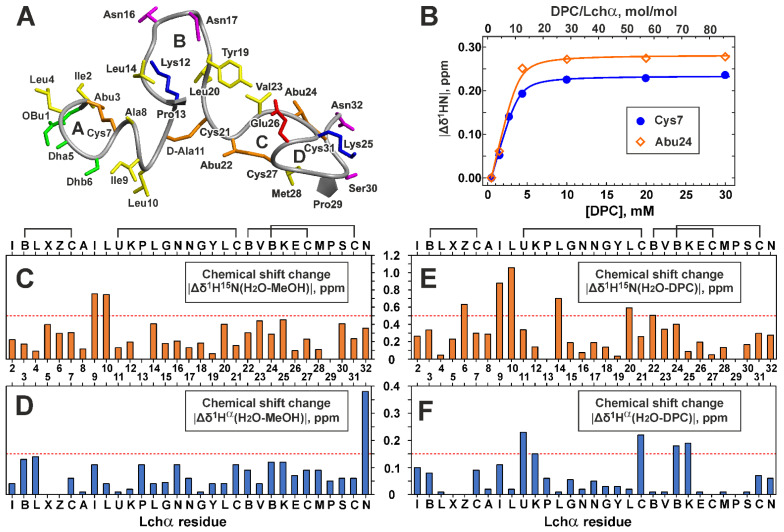
Figure 4.
Spatial structure of Lchα in methanol (A), Lchα binding to DPC micelles (B), and comparison of Lchα chemical shifts in different environments (C–F). (A) The representative conformer of Lchα in ribbon representation. The positively charged, negatively charged, hydrophobic/aromatic, polar, and non-standard residues are colored in blue, red, yellow, magenta, and green, respectively. The lanthionine and methyllanthionine bridges are colored orange. Thioether bridging rings are marked with the capital letters A, B, C, and D. (B) Titration of 0.35 mM Lchα sample by DPC. Titration curves for 1HN of Cys7 and Abu27 are approximated by Langmuir isotherm. (C–F) Changes in 1H15N and 1Hα chemical shifts upon Lchα transfer from water to methanol (C,D) and DPC micelles (E,F). The residue names are given in the one letter code format (see caption to Figure 3).