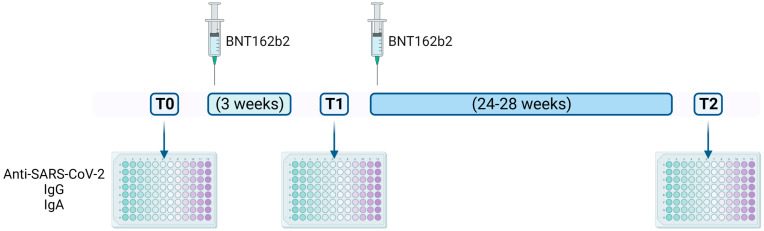
Figure 1.
Experimental design. During this observational study, serum samples were collected (1) before the first dose of BNT162b2 vaccine (T0); (2) three weeks later, before the administration of the second dose (T1); and (3) 24–28 weeks after T1 (T2). Neutralizing anti-SARS-CoV-2 spike protein IgG and IgA were quantified by ELISA at T0, T1 and T2. A questionnaire recording local and systemic adverse events for 1 week after each administration was provided to enrolled patients at T0 and T1 and collected in the next 2–4 weeks.