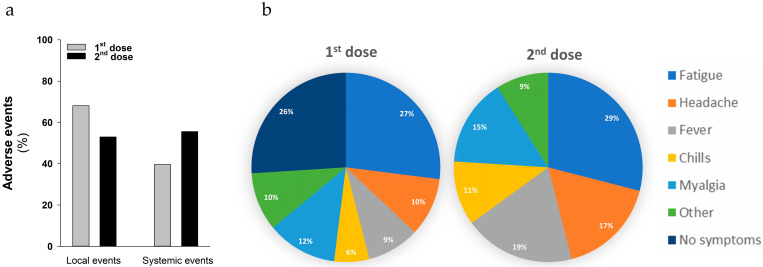
Figure 3.
Adverse events elicited by BNT162b2 vaccine in pwCF. (a) Percentage of local adverse events including local pain at the injection site and rashes or systemic adverse events upon vaccination in our cohort of 260 patients after the first dose (grey histogram) and the second dose (black histogram) administration. (b) Main systemic adverse events reported in pwCF after first and second dose administrations of vaccine are represented as pie charts. Incidence (%) of these adverse events is indicated within the graphs.