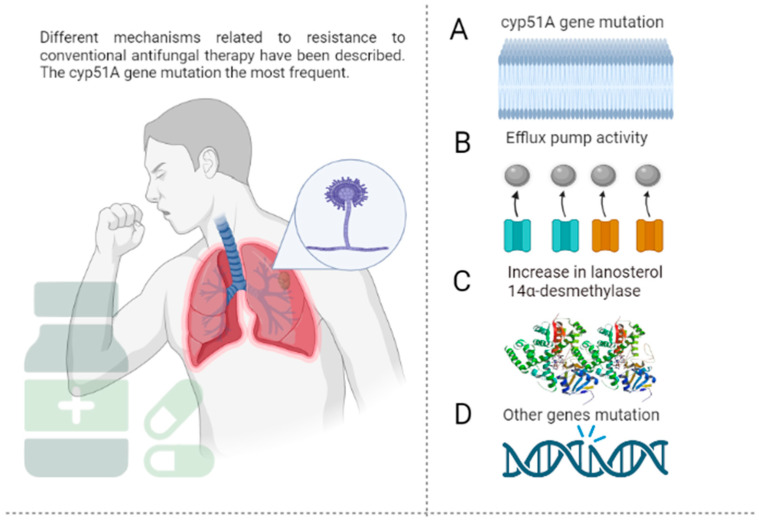
Figure 1.
Mechanisms of resistance of A. fumigatus to conventional antifungals. (A)—Resistance to azole antifungals is often derived from the mutation of the cyp51A gene, which codes lanosterol 14-α-demethylase, an important enzyme in ergosterol synthesis. More than 30 mutations have been identified, including the amino acid substitution Gly54, Pro216, Phe219, Met220, and Gly448. Resistance-associated loss-of-function mutations of ERG3 protect fungal cells from damage by the toxic 14α-methyl-3,6-diol product due to the accumulation of 14α-methylfecosterol that replaces ergosterol and leads to functional membranes, negating the action of azoles in the ergosterol biosynthetic pathway. (B)—The efflux pumps deliver the drug to the extracellular space, ensuring a lower concentration at the target site. This action is mediated by some protein superfamilies such as the ATP-binding cassette (ABC) and the major facilitator superfamily (MFS). (C)—Overexpression of ERG11 results in increased concentrations of lanosterol 14-α-demethylase and, consequently, higher amounts of the antifungal are required to inhibit the enzyme. (D)—Mutations different from cyp51A, such as cyp51B (which shares 59% of the cyp51A sequence), are less frequent, and their implications for azole resistance have not been extensively studied. The figure was created with https://app.biorender.com.