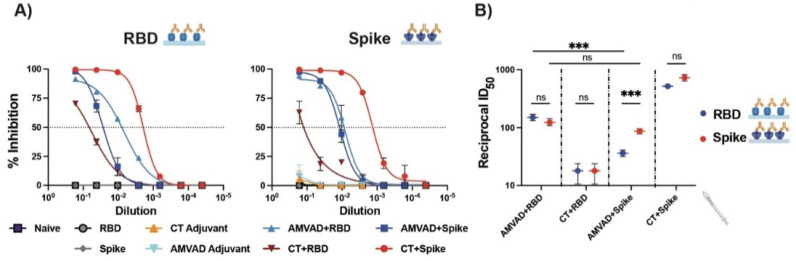
Figure 2.
Systemic neutralizing antibodies induced by the intranasal administration with adjuvanted RBD or spike antigens. (A) Measurement of relative inhibition of soluble ACE2 and immobilized RBD or spike interaction by neutralizing antibodies in plasma collected at endpoint by surrogate neutralization ELISA (snELISA). (B) Reciprocal ID50 neutralizing titer of adjuvanted vaccine formulations in blocking RBD (blue) or spike (red) and ACE2 ligand interaction. This assay is representative of technical triplicates and presented as mean ± standard deviation. Statistical significance was calculated by one-way ANOVA followed by Tukey correction; n.s.: no statistical difference; *** p ≤ 0.001. Individual mice samples were pooled and analyzed in triplicate.