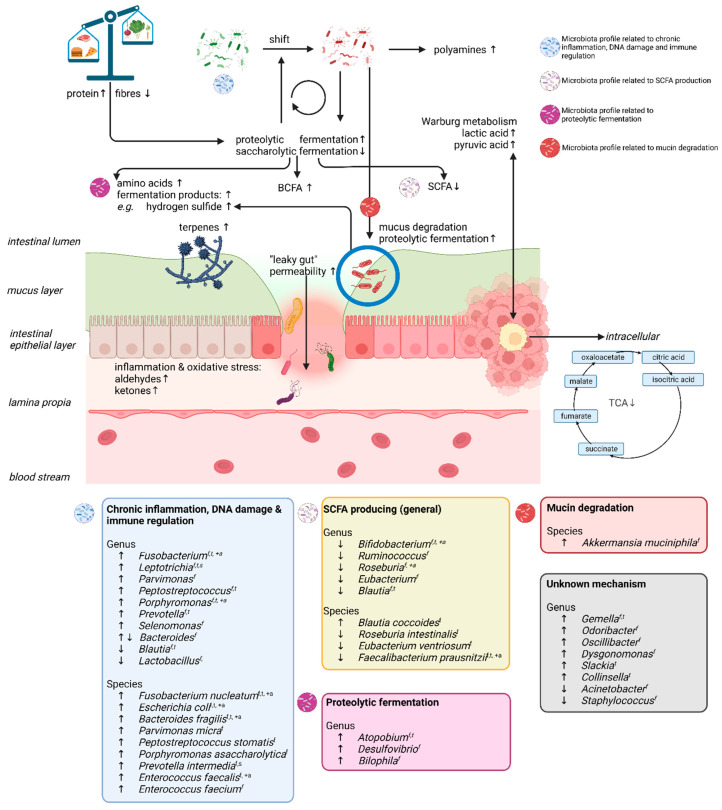
Figure 2.
A schematic overview of the volatolome and gut microbial microenvironment in colorectal neoplasia. Diets low in fiber and rich in proteins cause a decrease in saccharolytic fermentation and an increase in proteolytic fermentation. As a result, detrimental amino acids and proteolytic fermentation products (including branched chain fatty acids) increase in concentration while beneficial short chain fatty acids decrease in concentration. This temporary effect can become long-term by adhering to such a diet for longer periods, presumably by causing shifts in gut microbiota. Unfavorable microbes can degrade host glycans and mucins in the intestinal lumen, which have a function in maintaining gut barrier integrity. This cycle is self-empowering. Moreover, such unfavorable gut microbiota shifts may include an unfavorable mycobiome, causing a rise in terpenes. As a consequence, in addition to other immune-related factors, the intestinal barrier function is compromised, causing low-grade systematic oxidative stress and giving rise to enhanced aldehyde and ketone concentrations. In turn, microbes may produce amines for protection. In addition, the microbiota may also be involved in pathways that enhance Warburg metabolism and thus empower cancerous cells by providing energy for fast growth, as the aerobic tricarboxylic acid cycle is downregulated in cancerous cells. In this figure, only the volatolome was considered in relation to the microbiota. CRC-associated microbiota data is adapted from references [100,101,102,103]. Up arrows denote CRC or adenoma-enriched VOCs and gut microbiota; down arrows denote CRC or adenoma-depleted VOCs and gut microbiota. BCFA: branched chain fatty acid, SCFA: short chain fatty acid, TCA: tricarboxylic acid cycle, VOCs: volatile organic compounds, f = fecal sample, t = tissue sample, s = saliva sample, +a = also observed in colorectal adenomas.