Table 3.
TRPV3 antagonists.
| Entry | Name | Structure | Activate | Inhibit | IC50 for TRPV3 | Description/Use | Reference(s) |
|---|---|---|---|---|---|---|---|
| Natural compounds | |||||||
| 1 | Citrusinine II |
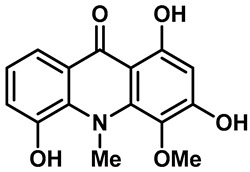
|
— | TRPV3 | 12.43 μM | Citrusinine II can selectively inhibit TRPV3 and reduce itchy behavior by interacting with Y564 in the TRPV3 helix. | [104] |
| 2 | Osthole |
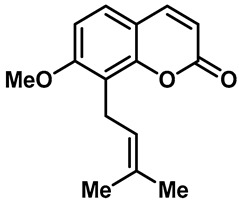
|
— | TRPV1 TRPV3 |
37.0 ± 1.9 μM | Cusson, isolated from Cnidium monnieri (L.), is used as an antipruritic herbal medicine. | [105,141] |
| 3 | Isochlorogenic acid A |
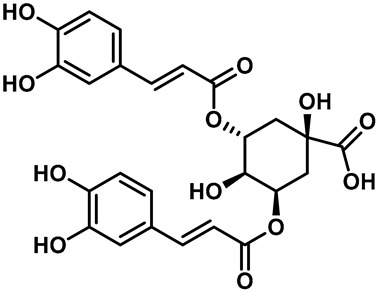
|
— | TRPV3 | 2.7 ± 1.3 μM | A TRPV3 specific inhibitor that can significantly reverse ear swelling in dermatitis and chronic pruritus. | [107] |
| 4 | Isochlorogenic acid B |
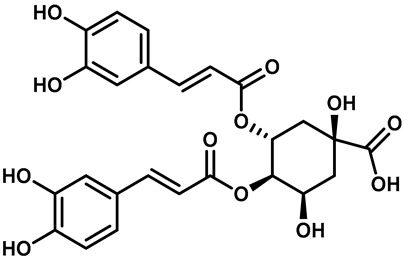
|
— | TRPV3 | 0.9 ± 0.3 μM | A TRPV3 specific inhibitor that can significantly reverse ear swelling in dermatitis and chronic pruritus. | [107] |
| 5 | Forsythoside B |
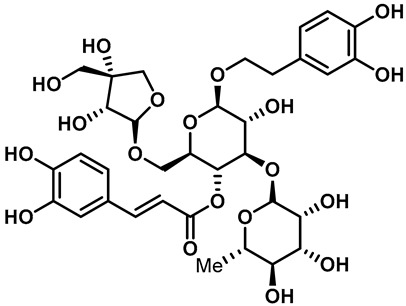
|
— | TRPV3 | 6.7 ± 0.7 μM | A TRPV3 inhibitor that can significantly reduce acute pruritus. | [108] |
| 6 | Monanchomycalin B |
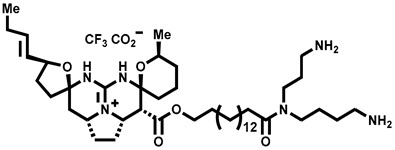
|
— | TRPV1 TRPV2 TRPV3 |
3.25 μM | A TRPV inhibitor that is isolated from the marine sponge Monanchora pulchra. | [112] |
| 7 | Verbascoside |
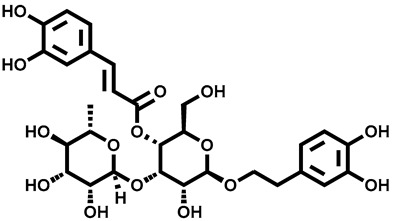
|
— | TRPV3 | 14.1 ± 3.3 μM | A TRPV3 inhibitor that can effectively relieve atopic dermatitis when applied topically. | [109] |
| 8 | Pulchranin A |
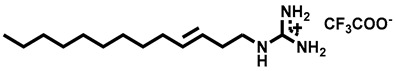
|
— | TRPV1 TRPV3 TRPA1 |
71.8 ± 9.4 μM | A moderately potent TRPV1 inhibitor and a minimally potent TRPV3 and TRPA1 inhibitor that is isolated from marine sponge Monanchora pulchra. | [110,111] |
| 9 | Pulchranin B |
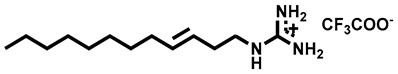
|
— | TRPV1 TRPV3 TRPA1 |
117.9 ± 11.8 μM | A moderately potent TRPV1 inhibitor and a minimally potent TRPV3 and TRPA1 inhibitor that is isolated from marine sponge Monanchora pulchra. | [110] |
| 10 | Pulchranin C |
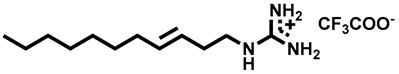
|
— | TRPV1 TRPV3 TRPA1 |
>200 μM | A moderate potent TRPV1 inhibitor and a minimally potent TRPV3 and TRPA1 inhibitor that is isolated from marine sponge Monanchora pulchra. | [110] |
| Synthetic compounds | |||||||
| 11 | Ruthenium Red |
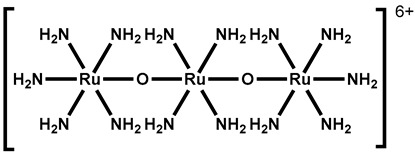
|
— | TRP(S) | NA | Broad-spectrum, non-selective, cationic channel blockers. | [113] |
| 12 | 2,2-diphenyltetrahydro-furan (DPTHF) |
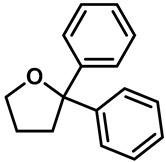
|
— | TRPV1 TRPV2 TRPV3 |
6–10 μM | 2-APB structural analogue. | [115] |
| 13 | PC5 |
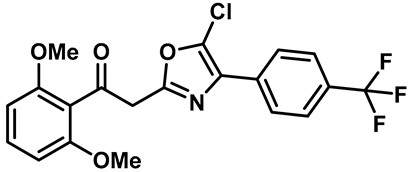
|
— | TRPV3 | 2.63 ± 0.28 μM | A TRPV3 inhibitor that is obtained by virtual protein structure screening and lead compound structure optimization. | [116] |
| 14 | 7C |
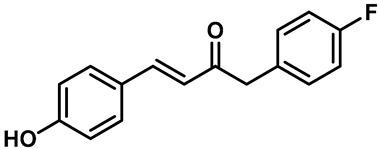
|
— | TRPV3 | 1.05 μM | A TRPV3 inhibitor that is obtained by the bioelectron isoarrangement principle. | [117] |
| 15 | Bupivacaine |
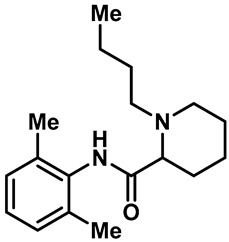
|
— | TRPV3 | 0.17 ± 0.04 mM | A TRPV3 inhibitor that can be used as local anesthetics by extracellular interactions of their charged forms with the TRPV3 channel pore. | [118] |
| 16 | Mepivacaine |
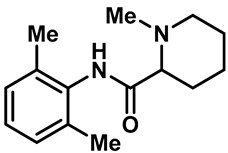
|
— | TRPV3 | 1.4 ± 0.3 mM | A TRPV3 inhibitor that can be used as local anesthetics by extracellular interactions of their charged forms with the TRPV3 channel pore. | [118] |
| 17 | Lidocaine |
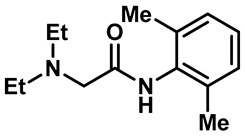
|
— | TRPV3 | 2.5 ± 0.5 mM | A TRPV3 inhibitor that can be used as local anesthetics by extracellular interactions of their charged forms with the TRPV3 channel pore. | [118] |
| 18 | Ropivacaine |
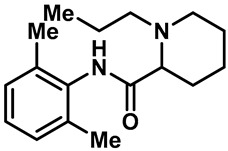
|
— | TRPV3 | 0.28 ± 0.04 mM | A TRPV3 inhibitor that can be used as local anesthetics by extracellular interactions of their charged forms with the TRPV3 channel pore. | [118] |
| 19 | Dyclonine |
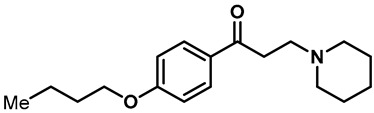
|
— | TRPV3 | 3.2 μM | A TRPV3 inhibitor that can ameliorate the hyperactivity caused by itch/scratching behaviors. | [119] |
| 20 | Example #64 (WO 2006/122156) |
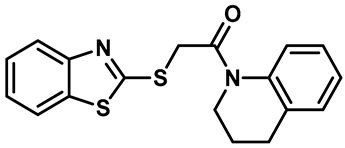
|
— | TRPV3 | 0.2–1 μM | A TRPV3 inhibitor discovered by Hydra Biosciences that can reduce heat sensitivity after carrageenan injection in burns or hind paws. | [121] |
| 21 | DCP-THQ (WO 2007/056124) |
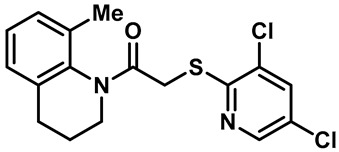
|
— | TRPV3 | 117 nM | A TRPV3 inhibitor discovered by Hydra Biosciences. | [123] |
| 22 | Example #19 (US 2010/0292554A1) |
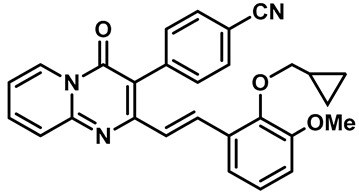
|
— | TRPV3 | 200–1000 nM | A TRPV3 inhibitor discovered by Glenmark. | [124] |
| 23 | Example #58 (US 2009/0286811A1) |
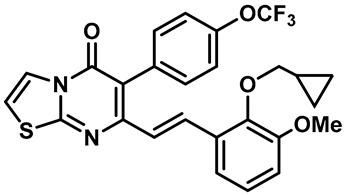
|
— | TRPV3 | < 500 nM | A TRPV3 inhibitor discovered by Glenmark. | [125] |
| 24 | Example #58 (WO 2009/130560) |
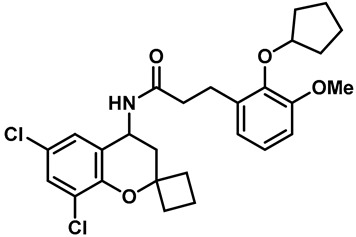
|
— | TRPV3 | <250 nM | A TRPV3 inhibitor discovered by Glenmark. | [126] |
| 25 | Example #23 (WO 2010/055384) |
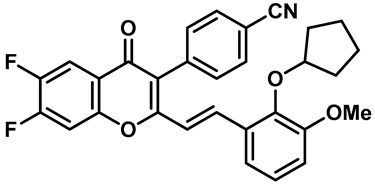
|
— | TRPV3 | <50 nM | A TRPV3 inhibitor discovered by Glenmark. | [127] |
| 26 | Example #7 (US2010/0152192) |
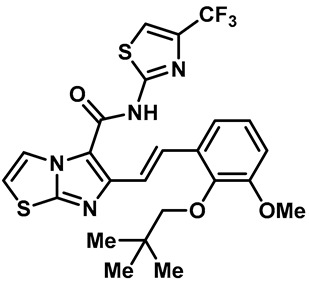
|
— | TRPV3 | <100 nM | A TRPV3 inhibitor discovered by Glenmark. | [128] |
| 27 | Example #37 (US2010/0152192) |
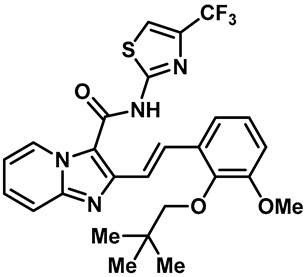
|
— | TRPV3 | <100 nM | A TRPV3 inhibitor discovered by Glenmark. | [128] |
| 28 | Example #83 (WO2010/073128) |
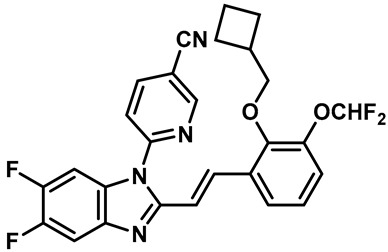
|
— | TRPV3 | <50 nM | A TRPV3 inhibitor discovered by Glenmark. | [129] |
| 29 | 74a |
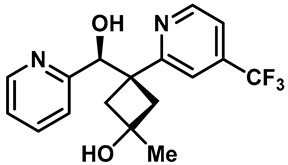
|
— | TRPV3 | 0.38 μM | A high potent TRPV3 inhibitor discovered by Glenmark that can be effective in rat neuropathic pain model. | [130] |
| Endogenous substance | |||||||
| 30 | 17R-RvD1 |
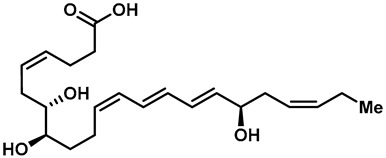
|
— | TRPV3 | 0.4 μM | An endogenous TRPV3 inhibitor of endogenous lipid metabolites. | [142] |
| 31 | Isopentenyl pyrophosphate (IPP) |
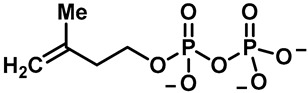
|
— | TRPV3 TRPA1 |
0.24 μM | An endogenous TRPA1 and TRPV3 inhibitor for topical analgesia. | [143] |
