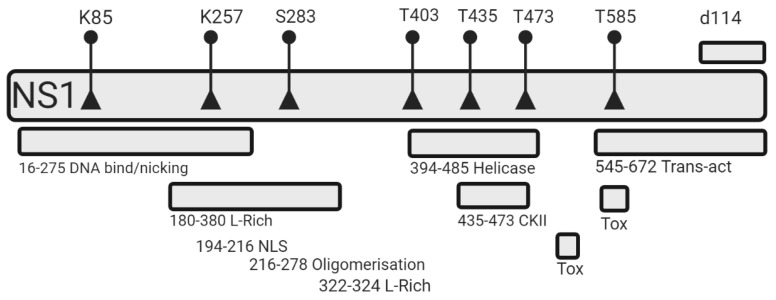
Figure 1.
Schematic overview of non-structural protein 1 (NS1) domains and mutation sites. The non-structural parvovirus-derived protein NS1 is comprised of 672 amino acids. Amino acid positions of previously described functional domains within the NS1 protein are summarized: A site-specific DNA binding domain involved in site- and strand-specific nicking (16–275), an L-Rich area which was shown to be crucial for NS1-mediated toxicity (180–380), a nuclear localization signal (194–216), a motif controlling self-assembly into oligomers (216–278), a helicase domain including a NTP-binding pocket (394–485), a region binding CKIIα (435–473), whose interaction is needed for many NS1- signaling pathways, two toxicity domains crucial for NS1 cytotoxicity, and a transactivator domain (545–672), which positively regulates the expression of viral proteins [22]. The locations of specific acetylation [15] and phosphorylation [13,14] sites, where single amino acid mutations and a deletion of 114 nucleotides (d114) [18] were introduced during this work, are indicated. Mutations were introduced by site-directed mutagenesis. For a review see Nüesch et al. [2,10,22].