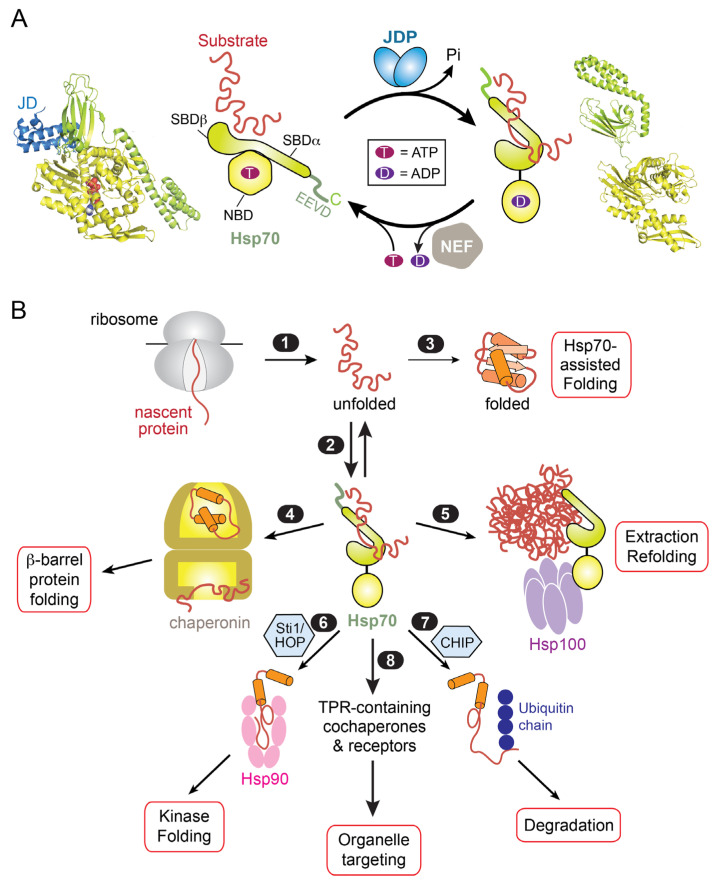
Figure 1.
Overview of the Hsp70 functional cycle and its role in protein biogenesis. (A) The ATPase cycle of Hsp70 regulates the capture and release of substrate proteins and is driven by its cochaperones J-domain proteins (JDPs) and nucleotide exchange factors (NEFs), which catalyze the hydrolysis of ATP and the release of ADP, respectively. The various domains in Hsp70 are indicated. The left panel shows the crystal structure of the J-domain (JD) of the bacterial JDP, DnaJ (blue), bound to the bacterial Hsp70 DnaK in the ATP-state (PDB# 5nro). The right panel shows the crystal structure of DnaK in the apo-state (PDB# 2kho). The SBD and NBD of DnaK are in lime and lemon, respectively. The bound ATP is in spacefill. (B) Hsp70 acts as a central hub in the cellular chaperone network to coordinate diverse protein biogenesis pathways, as described in the text.