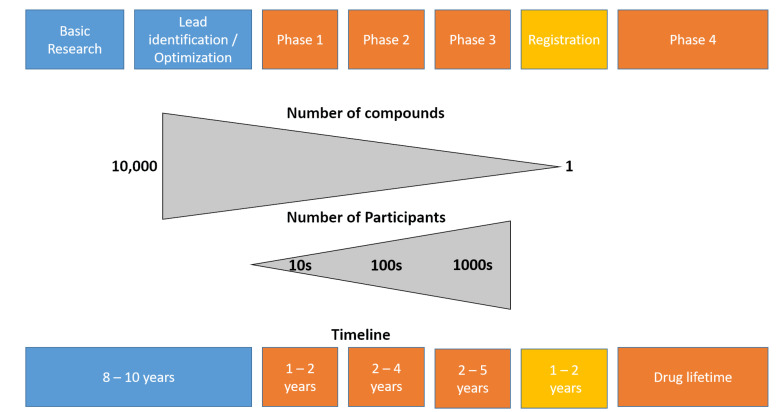
Figure 1.
The rocket model, adapted from Verweij et al., 2019 [11]. Note that this model and its numbers relate to the development of new drugs, as no data are available for new interventions in rehabilitation sciences. Blue colors indicate the different steps of the discovery, development and preclinical research, orange represents the clinical development. The different steps of the clinical development are as follows. Phase 1—healthy volunteer study: this is the first time the drugs is tested in people; less than 100 volunteers are usually involved, and the pharmacokinetics, absorption, metabolism, and excretion effects on the body, as well as any adverse effects associated with safe dose ranges, will be determined. Phase 2—small sample size study in patient population: Evaluates the safety and effectiveness of the medicine in an additional 100–500 patients who may receive a placebo or a previously utilized standard of care. The analysis of the ideal dosage strength aids in the development of schedules, while adverse events and dangers are documented. Phase 3—large-scale clinical study: typically enrolls 1000–5000 patients, allowing medication labeling and adequate drug usage instructions. Phase 3 studies need substantial cooperation, planning, and coordination and control on the part of an Independent Ethics Committee (IEC) or an Institutional Review Board (IRB) in preparation for full-scale manufacturing after medication approval.