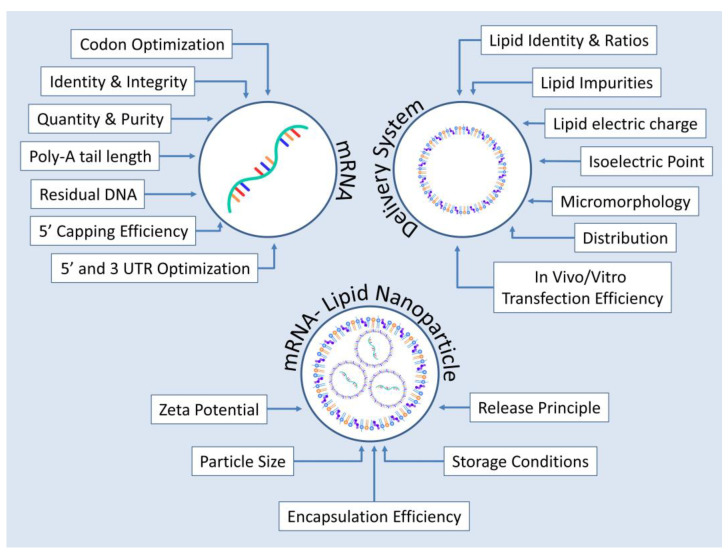
Figure 5.
Critical quality attributes in the production of encapsulated mRNA products to be used in humans for protein replacement therapies. Qualities examined are the ones pertaining to the mRNA molecule encoding the protein of interest, to the delivery system as well as to the finished product which in this case is an mRNA–LNP formulation.