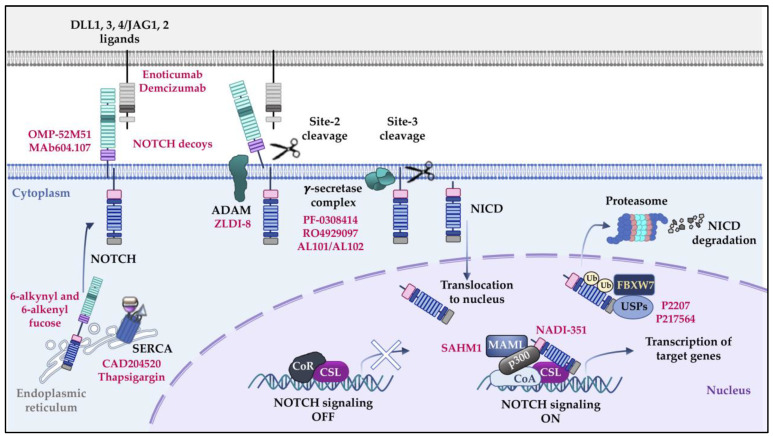
Figure 1.
NOTCH signaling and targeting strategies. Interaction of NOTCH receptors with DLL/JAG ligands exposes site-2 located within the HD domain of extracellular NOTCH, allowing proteolytic cleavage by ADAM metalloproteases. Sequential cleavage by a γ-secretase complex at the transmembrane region (site-3) of the receptor releases the NOTCH intracellular domain (NICD) that translocates to the nucleus and binds to the transcription factor CSL. In the absence of NOTCH signaling (NOTCH signaling OFF), CSL actively represses transcription of NOTCH target genes owing to interaction with corepressors (CoR). Upon NOTCH activation (NOTCH signaling ON), NICD recruits a transcriptional complex (including MAML, p300 and CoA) that displaces CoR from CSL, allowing CSL to become transcriptionally active and to induce NOTCH target gene transcription. Thereafter, FBXW7-dependent ubiquitination and subsequent proteasomal degradation of NICD lead to NOTCH signaling shutdown. NOTCH signaling can be efficiently inhibited by molecular targeting at different levels (red) by: (1) blocking receptor-ligand interactions with monoclonal antibodies (mAbs) [i.e., anti-NOTCH1 (OMP-52M51, Mab604.107) and anti-DLL4 (Enoticumab, Demcizumab) mAbs], or with NOTCH decoys; (2) inhibiting receptor proteolytic cleavage with ADAM inhibitors (ZLDI-8) or γ-secretase inhibitors (i.e., PF-0308414, RO4929097, AL101/AL102); (3) blocking NOTCH transcriptional complex activation with MAML1 inhibitors (SAHM1) or NOTCH ternary complex inhibitors (NADI-351); (4) favoring NOTCH degradation with USP7 inhibitors (P2207, P217564); or (5) interfering with NOTCH receptor maturation with POFUT1 inhibitors (6-alkynyl and 6-alkenyl fucose) and SERCA inhibitors (CAD204520, Thapsigargin).