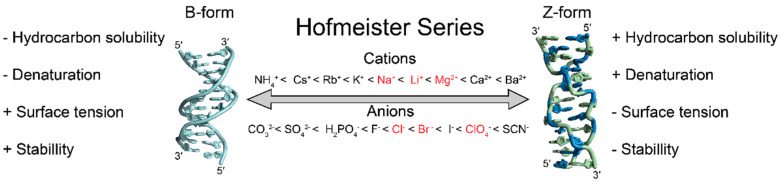
Figure 9.
Hofmeister salts stabilize the Z-conformation. Hofmeister salts influence macromolecular structures by affecting hydrocarbon solubility, denaturation, surface tension, and stability [105,106,107,108,109,110,111]. The B-to-Z transition has been shown to be promoted by Hofmeister salts, although the direct cause has not been directly studied. Contributing factors may be due to differences in solvent accessible surface area or hydrophobicity between the right- and left-handed conformations or by promoting the Z-conformation through destabilization of the right-handed conformation. Ions highlighted in red can be definitively placed for salts affecting the A-to-Z transition as well [3,87,103].