Abstract
Bacterial resistance refers to the ability of bacteria to resist the action of some antibiotics due to the development of adaptation and resistance mechanisms. It is a serious public health problem, especially for diseases caused by opportunistic bacteria. In this context, the search for new drugs, used alone or in combination, appears as an alternative for the treatment of microbial infections, and natural products, such as essential oils, are important in this process due to their structural diversity, which increases the probability for antimicrobial action. The objective of this study was to extract and identify the chemical components of the essential oil from Croton conduplicatus (EOCC), to evaluate the antimicrobial activity, to investigate the effect of the interaction between the EOCC and different antibiotics and to evaluate its antibiofilm potential. The EOCC was obtained by hydrodistillation. Based on chemical characterisation, 70 compounds were identified, with 1.8 cineole (13.15%), p-cymene (10.68%), caryophyllene (9.73%) and spathulenol (6.36%) being the major constituents. The minimum inhibitory concentration (MIC) values of EOCC were 256 and 512 µg mL−1 for methicillin-sensitive and -resistant Staphylococcus aureus strains (MSSA and MRSA), respectively. The combinations of EOCC with the antibiotics oxacillin and ampicillin were synergistic (OXA/EOCC and AMP/EOCC combined decreased the OXA MIC and AMP MIC to 0.5 and 0.25 for MSSA, respectively, and OXA/EOCC and AMP/EOCC combined decreased the OXA MIC and the AMP MIC to 1 and 0.5 for MRSA, respectively) and could modify the resistance profile of MSSA and MRSA strains. The results indicated that EOCC was also able to partially inhibit biofilm formation. Our study presents important information about the chemical composition of EOCC and its antimicrobial potential and provides a reference to determine the mechanisms of action of EOCC and its use in pharmaceutical formulations.
Keywords: Croton genus, knife breaker, volatile compounds, Staphylococcus aureus, resistance profile, antibiofilm
1. Introduction
Staphylococcus aureus is a Gram-positive, opportunistic bacterial species characterised by grouped cocci and clusters of cocci that are found mainly in the nasal microbiota. They can cause an infectious condition when they get into the bloodstream by breaking through mucous membrane or skin tissue [1]. This species is highly pathogenic, virulent and shows considerable resistance to environmental factors. A major concern worldwide is the capacity for multi-resistance to antibacterial agents used to combat Gram-positive bacterial infections, such as beta-lactams, glycopeptides and oxazolidones. One of the bacterial resistance profiles of great concern today are those of methicillin-resistant S. aureus (MRSA) strains. Generally, MRSA infections are linked with increased difficulty of treatment, morbidity, mortality and high costs to health services [2,3].
The problems related to MRSA strains become more severe when this pathogen develops a biofilm, a structure formed by polymeric substances, proteins and extracellular DNA [4]. This biofilm can form on the surface of medical materials intended for surgical application. Thus, with high resistance to antimicrobials, MRSA strains contaminate patients to the point of making their treatment difficult, increasing life risks, hospital stay length and, consequently, costs. [5]. In view of the complications related to the treatment of S. aureus and its resistant strains, The World Health Organization has published a list classifying methicillin-sensitive S. aureus (MSSA) and MRSA as high-priority pathogens in the search for new compounds to aid therapy [6].
Due to the increase in bacterial resistance, the development of new bioactive compounds is one of the strategies used in the search for products that may have activity against these pathogens [7,8]. Essential oils (EOs) have emerged as an alternative to combat these microorganisms. Normally, these EOs are a mixture of phenylpropanoids or terpenes (monoterpenes, sesquiterpenes and/or diterpenes), which have different chemical functions, such as alcohols, ketones, aldehydes, and that can present a diverse range of biological activities, including antibacterial activity [9].
Several studies evidence the inhibition of MSSA and MRSA strains by EOs and the synergistic relationship between EOs and antibacterials, highlighting their efficiency [3,10,11].
The genus Croton, belonging to the family Euphorbiaceae, contains species that produce EO [12]. Croton conduplicatus is a native plant species of the Brazilian caatinga and widely used in folk medicine to combat headaches, indigestion and influenza [13]. In a previous study, the essential oil obtained from fresh leaves of C. conduplicatus was evaluated by gas chromatography and mass spectrometry (GC-MS), and 42 chemical compounds were identified, of which 1,8-cineole and p-cymene were the major compounds. This EO showed anxiolytic, sedative and antinociceptive activities in an in vivo study using mice [14].
Several studies show that 1,8-cineole and p-cymene have antibacterial potential and that can reduce the resistance of MRSA and MSSA strains against antimicrobials [15,16,17,18]. Thus, the objective of this study was to carry out the extraction and chemical characterisation of the EO from the dried leaves of C. conduplicatus by GC-MS, to evaluate the antimicrobial activity of the EO from C. conduplicatus (EOCC) against strains of S. aureus sensitive to methicillin (MSSA), methicillin-resistant S. aureus (MRSA) and other microorganisms and to investigate the in vitro interaction of EOCC with conventional antimicrobials against MSSA and MRSA strains and its antibiofilm activity.
2. Results
2.1. Chemical Characterisation of C. conduplicatus Essential Oil
Essential oils have a high concentration of bioactive compounds such as terpenes, sesquiterpenes, phenolic compounds, phenylpropanoids, non-terpenic aliphatic compounds and heterocyclic compounds, which are responsible for the biological activity of these oils.
Analysis of the chemical composition of the EOCC was performed by GC-MS. Table 1 shows the chemical composition, the retention indices, the retention time, and the relative percentage of each constituent present in EOCC.
Table 1.
Chemical constituents of Croton conduplicatus essential oil.
| No | Compounds a | RILit b | RICalc c | RT (min) | Area (%) | No | Compounds a | RILit b | RICalc c | RT (min) | Area (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | α-Tricyclene | 926 | 952 | 7.02 | 0.09 | 37 | α-Cubebene | 1348 | 1340 | 17.33 | 1.19 |
| 2 | α-Thujene | 930 | 955 | 7.08 | 0.57 | 38 | β-Bourbonene | 1388 | 1350 | 17.61 | 0.20 |
| 3 | α-Pinene | 939 | 960 | 7.22 | 4.93 | 39 | β–Elemene | 1390 | 1355 | 17.74 | 1.92 |
| 4 | 1-Ethylbutyl Hydroperoxide d | - | 966 | 7.38 | 0.21 | 40 | Caryophyllene | 1419 | 1397 | 18.85 | 9.73 |
| 5 | Camphene | 954 | 970 | 7.50 | 0.73 | 41 | β-Copaene | 1432 | 1409 | 19.16 | 0.31 |
| 6 | Sabinene | 975 | 986 | 7.92 | 1.25 | 42 | cis-Eudesma-6,11-diene | 1477 | 1424 | 19.56 | 0.62 |
| 7 | β-Pinene | 979 | 990 | 8.02 | 2.77 | 43 | α-Caryophyllene | 1454 | 1442 | 20.05 | 1.77 |
| 8 | β-Myrcene | 990 | 997 | 8.21 | 0.33 | 44 | Alloaromadedrene | 1460 | 1448 | 20.20 | 1.99 |
| 9 | 2,3-dihydro-1,8-cineole | 991 | 999 | 8.27 | 0.05 | 45 | γ-Gurjunene | 1477 | 1466 | 20.68 | 0.27 |
| 10 | α-Phellandrene | 1002 | 1012 | 8.60 | 3.08 | 46 | Germacrene D | 1485 | 1474 | 20.90 | 2.64 |
| 11 | α-Terpinene | 1017 | 1021 | 8.85 | 0.20 | 47 | β-Selinene | 1490 | 1484 | 21.17 | 0.87 |
| 12 | p-Cymene | 1024 | 1028 | 9.04 | 10.68 | 48 | Bicyclogermacrene | 1500 | 1493 | 21.40 | 3.40 |
| 13 | D-Limonene | 1029 | 1033 | 9.16 | 1.51 | 49 | α-Muurolene | 1500 | 1496 | 21.48 | 0.72 |
| 14 | β-Thujene d | - | 1034 | 9.20 | 0.69 | 50 | Eremophila-1(10),8,11-triene d | - | 1502 | 21.65 | 0.16 |
| 15 | 1,8-Cineole | 1031 | 1037 | 9.26 | 13.15 | 51 | Germacrene A | 1509 | 1507 | 21.77 | 0.42 |
| 16 | β-cis-Ocimene | 1037 | 1048 | 9.55 | 0.18 | 52 | γ-Cadiene | 1513 | 1513 | 21.95 | 0.43 |
| 17 | γ-Terpinene | 1059 | 1060 | 9.89 | 0.40 | 53 | δ-Cadinene d | - | 1520 | 22.13 | 1.04 |
| 18 | Cis-Sabinene hydrate | 1070 | 1073 | 10.22 | 0.18 | 54 | β-Calacorene | 1545 | 1548 | 22.86 | 0.48 |
| 19 | Isoterpinolene | 1088 | 1087 | 10.59 | 0.16 | 55 | Ciclohexane,1,3-diisopropenyl-6-methyl d | - | 1554 | 23.04 | 1.34 |
| 20 | Linalool | 1096 | 1099 | 10.91 | 2.39 | 56 | Germacrene B | 1561 | 1569 | 23.43 | 0.63 |
| 21 | cis-4-Thujanol | 1098 | 1101 | 10.98 | 0.18 | 57 | Cis-α-Copaene-8-ol d | - | 1574 | 23.56 | 0.51 |
| 22 | Cis-p-Menth-2-en-1-ol | 1121 | 1122 | 11.54 | 0.17 | 58 | Spathulenol | 1578 | 1591 | 24.02 | 6.36 |
| 23 | Trans-pinocarveol | 1139 | 1139 | 11.98 | 0.33 | 59 | Ledol | 1602 | 1622 | 24.85 | 0.50 |
| 24 | (+)-Camphor | 1146 | 1142 | 12.07 | 0.75 | 60 | Humulene epoxide II | 1608 | 1629 | 25.02 | 0.43 |
| 25 | Pinocarvone | 1164 | 1155 | 12.41 | 0.45 | 61 | β-Guayene d | - | 1635 | 25.19 | 0.44 |
| 26 | Terpineol <cis-dihydro-a-> | 1164 | 1161 | 12.57 | 0.23 | 62 | γ-Maaliene d | - | 1643 | 25.39 | 0.12 |
| 27 | Borneol | 1165 | 1163 | 12.63 | 0.67 | 63 | Epicubebol d | - | 1648 | 25.53 | 0.26 |
| 28 | Terpinen-4-ol | 1177 | 1170 | 12.81 | 1.44 | 64 | β-Spathulenol d | 1578 | 1655 | 25.73 | 0.60 |
| 29 | p-Cymen-8-ol | 1182 | 1174 | 12.92 | 0.19 | 65 | Bicyclo[7.2.0]undecan-3-ol, 11,11-dimethyl-4,8-bis(methylene)- d | - | 1660 | 25.85 | 0.34 |
| 30 | α-Terpineol | 1188 | 1181 | 13.11 | 1.96 | 66 | 10-epi-α –Cadinol | 1640 | 1665 | 25.99 | 0.88 |
| 31 | Cis-sabinol d | - | 1188 | 13.29 | 0.40 | 67 | α-Muurolol | 1646 | 1670 | 26.13 | 0.15 |
| 32 | Cis-piperitol | 1196 | 1192 | 13.40 | 0.09 | 68 | Epi-α-Muurolol | 1642 | 1681 | 26.40 | 0.72 |
| 33 | β-Sabinyl Acetate d | - | 1216 | 14.04 | 0.22 | 69 | Xantoxyline | 1668 | 1690 | 26.65 | 0.65 |
| 34 | Bornyl acetate | 1288 | 1250 | 14.94 | 0.36 | 70 | (1R,7S,E)-7-isopropyl-4,10-dimethylene-cyclodec-5-enol | 1686 | 1713 | 27.26 | 0.14 |
| 35 | Thymol | 1290 | 1260 | 15.21 | 0.53 | 71 | NI e | - | 1762 | 28.58 | 0.14 |
| 36 | α-Longipinene | 1352 | 1333 | 17.15 | 0.34 | Total identified | 95.94 |
a Constituents listed in order of elution on Elite-5MS column; b RILit. = Literature retention index [19]; c RICalc. = calculated retention index; d Compounds identified by NIST; e Not identified.
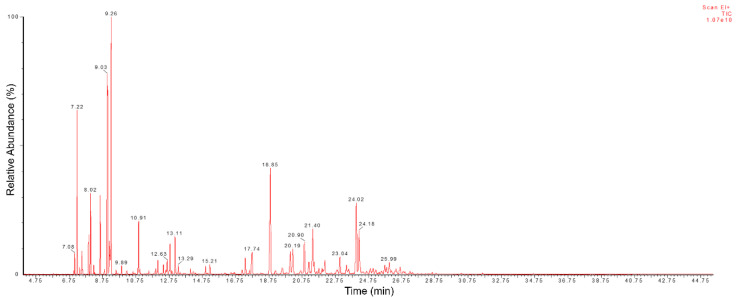
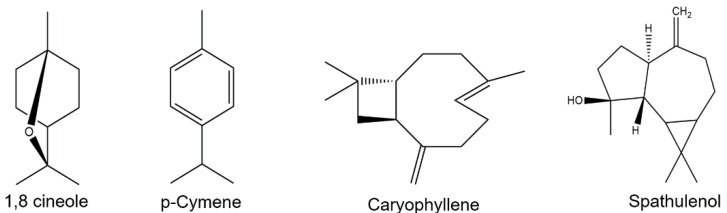
We verified the presence of 74 distinct peaks, as shown in the Figure 1, of which 70 were identified (Table 1), accounting for 95.94% of the chemical composition of EOCC. The monoterpenes 1,8-cineole (13.15%) and p-cymene (10.68%) and the sesquiterpenes caryophyllene (9.73%) and spathulenol (6.36%) were the major compounds (Figure 2). The other compounds with percentages below 5% were considered minor, such as α-pinene (4.93%), bicyclogermacrene (3.40%), α-phellandrene (3.08%), β-pinene (2.77%) and linalool (2.39%).
Figure 1.
GC-MS total ion chromatogram of C. conduplicatus essential oil.
Figure 2.
Chemical structures of the major compounds identified in C. conduplicatus essential oil via GC-MS analysis.
Among the major compounds 1,8-cineole, p-cymene, caryophyllene and spathulenol were identified by other authors as part of the main constituents of the chemical composition of the EO from C. conduplicatus [20,21,22,23,24]. In their results, these compounds showed similar average concentrations to those found here, with the exception of 1,8-cineole, which showed a concentration of 24.09% [21]. In addition, the compounds bicyclogermacrene and α-phellandrene were identified here as minor compounds, whereas in other studies, they were among the major constituents [20,21,22]. Differences in the number and identity of compounds were also identified in the GC-MS analyzes performed by [14] which revealed the presence of 50 peaks and 42 compounds identified in the EOCC obtained from fresh leaves of C. conduplicatus. The monoterpenes 1,8-cineol (21.42%) and p-cymene (12.41%) and the sesquiterpenes spathulenol (15.47%) and caryophyllene oxide (12.15%) were considered the majority constituents of the sample (Table 2). In our study, it was possible to identify and quantify 70 different chemical compounds, of which the monoterpenes 1,8 cineole (13.15%) and p-cymene (10.68%) were the majority, as well as the sesquiterpenes caryophyllene (9.73%) and spathulenol (6.36%). This variation in the chemical composition of the EOCC may be related to factors such as the specific location of leaf collection, growing season, botanical origin, climatic factors [25] and the drying process that was used on the plant material.
Table 2.
Comparison of chemical compounds obtained by GC-MS from the essential oil of fresh. leaves of C. conduplicatus (Oliveira Júnior et al., 2018).
| Peak | Compounds | RT (min) | % GC-MS |
|---|---|---|---|
| 1 | Tricyclene | 8.447 | 0.08 |
| 2 | α-Thujene | 8.726 | 0.50 |
| 3 | α-Pinene | 8.927 | 2.30 |
| 4 | Camphene | 9.465 | 0.49 |
| 5 | Sabinene | 10.521 | 1.46 |
| 6 | α-Phellandrene | 11.731 | 1.44 |
| 7 | p-Cymene | 12.574 | 12.41 |
| 8 | 1,8-Cineole | 12.792 | 21.42 |
| 9 | NI | 13.694 | 0.07 |
| 10 | γ-Terpinene | 13.942 | 0.14 |
| 11 | Terpinolene | 15.087 | 0.05 |
| 12 | (E)-Sabinene | 15.569 | 0.03 |
| 13 | NI | 15.716 | 0.13 |
| 14 | (Z)-p-Menth-2-en-1-ol | 16.402 | 0.16 |
| 15 | α-Campholenal | 16.535 | 0.01 |
| 16 | (E)-Pinocarveol | 16.977 | 0.18 |
| 17 | Camphor | 17.117 | 0.32 |
| 18 | Pinocarvone | 17.842 | 0.09 |
| 19 | Borneol | 17.989 | 0.52 |
| 20 | NI | 18.130 | 0.05 |
| 21 | Terpinen-4-ol | 18.417 | 2.28 |
| 22 | α-Terpineol | 19.001 | 0.60 |
| 23 | Isobornyl acetate | 22.236 | 0.32 |
| 24 | α-Copaene | 25.167 | 0.20 |
| 25 | β-Bourbonene | 25.450 | 0.21 |
| 26 | β-Elemene | 25.713 | 0.34 |
| 27 | (E)-Caryophylene | 26.560 | 7.52 |
| 28 | α-Humulene | 27.613 | 1.55 |
| 29 | Alloaromadendrene | 27.841 | 1.69 |
| 30 | Germacrene D | 28.473 | 0.31 |
| 31 | β-Selinene | 28.628 | 0.32 |
| 32 | Bicyclogermacrene | 28.955 | 1.61 |
| 33 | δ-Amorphene | 29.488 | 0.58 |
| 34 | δ-Cadinene | 29.776 | 0.53 |
| 35 | α-Calacorene | 30.355 | 0.14 |
| 36 | NI | 30.611 | 0.32 |
| 37 | Spathulenol | 34.413 | 15.47 |
| 38 | Caryophyllene oxide | 31.541 | 12.15 |
| 39 | Ledol | 32.105 | 1.50 |
| 40 | Humulene epoxide | 32.265 | 1.42 |
| 41 | Cubenol | 32.450 | 0.20 |
| 42 | Acorenol | 32.826 | 0.25 |
| 43 | NI | 32.966 | 0.44 |
| 44 | NI | 33.069 | 1.19 |
| 45 | Epi-α-Cadinol | 33.185 | 4.34 |
| 46 | α-Muurolol | 33.352 | 0.50 |
| 47 | β-Eudesmol | 33.449 | 0.51 |
| 48 | α-Cadinol | 33.578 | 1.02 |
| 49 | NI | 33.881 | 0.45 |
| 50 | NI | 35.751 | 0.15 |
| Total identified | 97.2 | ||
RT (min) = Retention times of the compounds; % GC-MS. = Relative percentage of the compound in the EOCC; NI = Not identified.
2.2. Antimicrobial Activity of C. conduplicatus Essential Oil
The EOCC showed antibacterial activity, with an minimum inhibitory concentration (MIC) of 256 µg mL−1 for the MSSA strain and 512 µg mL−1 for the MRSA strain (Figure 3). The bactericidal effect of EOCC was observed only in the presence of twice the MIC concentration. No antimicrobial activity of EOCC was observed against E. coli, P. aeruginosa and C. albicans strains because the MIC values against these strains were > 1024 µg mL−1 (Table 3). The MRSA strain used in this study presented a resistance profile to oxacillin (OXA) and ampicillin (AMP), which was detected through the determination of the MIC, highlighting the MIC of OXA of 32 µg mL−1, which is used for the detection of methicillin resistance, according to the breakpoints defined by the CLSI document M100 [26]. Because EOCC showed activity against MRSA and MSSA strains, these were selected for the in vitro combination step with antibiotics to investigate the synergistic effect and reduction in MIC of both the antibiotic and EOCC.
Figure 3.
Antimicrobial activity of C. conduplicatus essential oil relieved by the addition of 0.01% resazurin solution. Each set of three lines on the plate represents a tested microorganism, with the following strains: methicillin-sensitive S. aureus (MSSA), methicillin-resistant S. aureus (MRSA), Escherichia coli and Pseudomonas aeruginosa, respectively.
Table 3.
MIC/MBC or MFC of C. conduplicatus essential oil.
| Microrganisms | MIC/MBC or MFC (µg mL−1) | ||||
|---|---|---|---|---|---|
| EOCC | AMP | OXA | POL | ANF | |
| S. aureus ATCC 25923 (MSSA) | 256/512 | 2/4 | 2/4 | - | - |
| S. aureus ATCC 33591 (MRSA) | 512/1024 | 16/32 | 32/64 | - | - |
| E. coli ATCC 25922 | na | nd | - | - | - |
| P. aeruginosa ATCC 27853 | na | - | - | 1/1 | - |
| C. albicans ATCC 10231 | na | - | - | - | 0.5/1 |
ATCC—American Type Culture Collection; MIC—minimum inhibitory concentration; MBC—minimum bactericidal concentration; MFC—minimum fungal concentration; EOCC—essential oil from Croton conduplicatus; AMP—ampicillin; OXA—oxacillin; POL—polimixin B; ANF—anfotericin B; MSSA—methicillin-sensitive Staphylococcus aureus; MRSA—methicillin-resistant Staphylococcus aureus; na—no activity; nd—not determined.
2.3. Synergistic Activity of C. conduplicatus Essential Oil with Oxacillin and Ampicillin against S. aureus
A synergistic effect of combining subinhibitory concentrations of EOCC (≤ 1/2 MIC) with OXA and AMP was observed against MSSA and MRSA (Figure 4a,b) strains, with MIC reduction percentages ranging from 75% to 96.9%; the synergistic effect was determined by fractional inhibitory concentration (FICi) values that ranged from 0.0938 to 0.3125. Percentage reductions in the MIC of EOCC alone were also observed when combined with OXA and AMP. Thus, the EOCC showed potential to reduce OXA and AMP MIC, and these antibiotics showed potential to reduce the MIC of EOCC (Table 4).
Figure 4.
Checkerboard assay between C. conduplicatus essential oil with oxacillin (a) and C. conduplicatus essential oil with ampicillin (b) against the MRSA strain. The blue colour indicates the activity of the combined concentration.
Table 4.
Combination testing of C. conduplicatus essential oil with antimicrobials agents against MSSA and MRSA strains.
| S. aureus | Combination | Individual MIC (µg mL−1) | Combined MIC (µg mL−1) | Individual FIC | FIC Index (FICi) | MIC Reduction (%) | Combination Effect |
|---|---|---|---|---|---|---|---|
|
S. aureus ATCC 25923 (MSSA) |
OXA/EOCC | 2/256 | 0.5/16 | 0.25/0.0625 | 0.3125 | 75.0/93.75 | Synergistic |
| AMP/EOCC | 2/256 | 0.25/16 | 0.125/0.0625 | 0.1875 | 87.5/93.75 | Synergistic | |
|
S. aureus ATCC 33591 (MRSA) |
OXA/EOCC | 32/512 | 1/32 | 0.0313/0.0625 | 0.0938 | 96.9/93.75 | Synergistic |
| AMP/EOCC | 16/512 | 0.5/32 | 0.0313/0.0625 | 0.0938 | 96.9/93.75 | Synergistic |
ATCC—American Type Culture Collection; MSSA—methicillin-sensitive Staphylococcus aureus; MRSA—methicillin-resistant Staphylococcus aureus; OXA—oxacillin; AMP—ampicillin; MIC—minimum inhibitory concentration; FIC—fractional inhibitory concentration.
The combinations of EOCC with OXA and AMP were able to reverse resistance to these antibiotics, indicating that at concentrations lower than MIC, OXA and AMP became active against the strains when combined with EOCC.
For the MSSA strains, we verified a reduction in the MIC of both EOCC and OXA and AMP, indicating that EOCC in subinhibitory concentration in combination with these antibiotics has the potential to reduce the MIC for both methicillin-sensitive and methicillin-resistant strains (Table 4). Because of the in vitro combination tests using EOCC with the antibiotics OXA and AMP, it can be stated that these interactions had a synergistic effect.
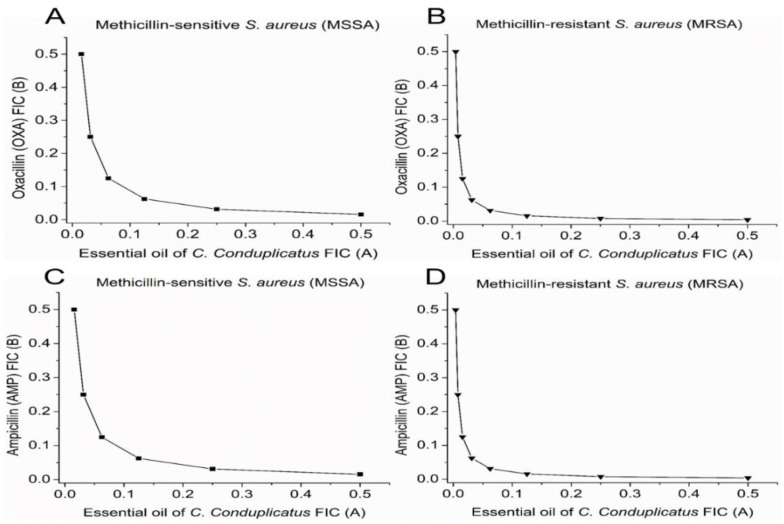
The results for MSSA and MRSA strains in terms of sensitivity and resistance to methicillin, respectively, are shown in Figure 1. For the MSSA strain, the MIC of isolated OXA was 2 µg mL−1. In combination with EOCC, the MIC of OXA was reduced to 0.5 µg mL−1, changing its profile from resistant to sensitive to this antibiotic. The reduction in OXA MIC was also observed in the MRSA strain (Figure 5B). A similar effect was observed in the associations of EOCC with AMP, with a synergistic effect against the MRSA strain, with a reduction in the MIC of AMP from 16 to 0.5 µg mL−1 (Figure 5D).
Figure 5.
Isobole curves showing the synergistic effect of essential oil from C. conduplicatus leaves with (A,B) oxacillin and (C,D) ampicillin against methicillin-sensitive Staphylococcus aureus (MSSA) ATCC 25923 and methicillin-resistant Staphylococcus aureus (MRSA) ATCC 33591, respectively.
2.4. Antibiofilm Activities of C. conduplicatus Essential Oil
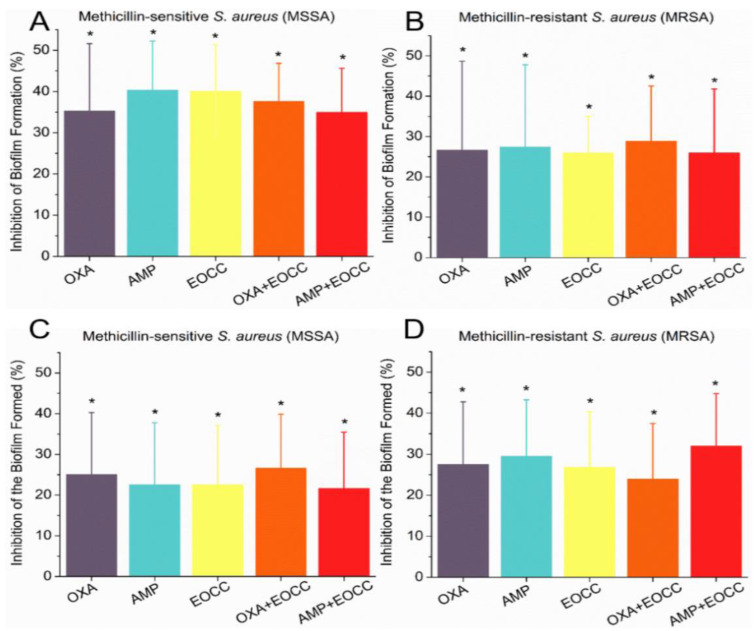
The antibiofilm activity of EOCC alone (at an concentration equal to the MIC) and in combination with OXA and AMP was measured against MRSA and MSSA strains. The percentages of biofilm inhibition reducing formed biofilms were evaluated (Figure 6). Figure 7 shows the antibiofilm activity of OECC and the combinations with OXA and AMP against the MRSA strain.
Figure 6.
Percentage inhibition of biofilm formation by EOCC, OXA and AMP alone and in combination against the MSSA strain (A); percentage inhibition of biofilm formation by EOCC, OXA and AMP in combination and alone against the MRSA strain (B); percentage inhibition of mature biofilm for EOCC, OXA and AMP alone and in combination against the MSSA strain (C); percentage inhibition of mature biofilm for EOCC, OXA and AMP alone and in combination against the MRSA strain (D). The asterisk indicates a non-significant difference (p > 0.05) according to the t-test.
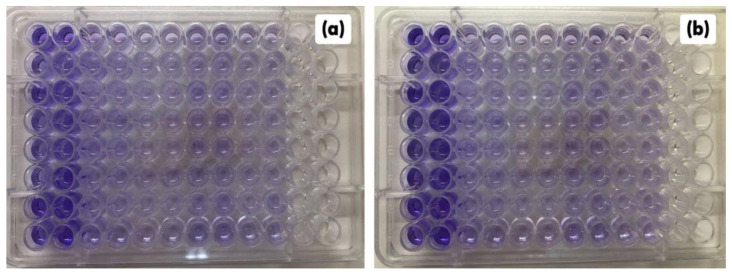
Figure 7.
Biofilm biomass stained with violet crystal after experiments of inhibition of biofilm formation (a) and activity against mature biofilm (b) for EOCC, OXA and AMP alone and in combination against the MRSA strain. The first two columns (1 and 2) in (a,b) represent the biomass of untreated biofilm; the columns 3 to 10 are the treatments with EOCC and the combinations with OXA and AMP.
The isolated EOCC at MIC showed inhibited biofilm formation in MSSA and MRSA strains by 18% and 22%, respectively. In the evaluation of the ability of EOCC to reduce formed (mature) biofilms of these strains, isolated EOCC was able to reduce the biofilm of the MSSA strain by 32% and that of the MRSA strain by 27%, highlighting the inhibition or reduction of biofilms by EOCC.
The EOCC combined with the antibiotics OXA and AMP, in subinhibitory concentrations, also showed activity, for most of the combinations, against the tested strains, with emphasis on the reduction of mature biofilm by the combination EOCC/OXA that was statistically equal (p > 0.05) the inhibition caused by EOCC (MIC concentration) against the strain MSSA and, the combination EOCC/OXA was also able to reduce the biofilm formed by the strain MRSA.
An inhibition of biofilm formation by EOCC and its combinations with OXA and AMP at concentrations lower than the MIC was also observed against the S. aureus strains tested, which was more pronounced for the combination EOCC/AMP against the MRSA strain.
The treatments applied, both regarding the inhibition of biofilm formation and of mature biofilm, showed differences only to the positive control used, indicating that the treatments were effective against MSSA and MRSA antibiofilm activities. Although there was no significant difference between the treatments, the results indicate that EOCC combined with OXA or AMP at subinhibitory concentrations has an effect similar to that of EOCC alone at a higher concentration (equivalent to the MIC).
3. Discussion
The chemical composition of the essential oil from C. conduplicatus leaves was obtained via GC-MS. Based on the results, it is rich in bioactive compounds, mainly monoterpenes and sesquiterpenes. Generally, EOs are natural products rich in bioactive compounds, such as monoterpenes and phenylpropanoids. These compounds are used by plants as a defense against predators and microorganisms, including those that are pathogenic to humans [27].
Studies indicate that the EOs from plants belonging to the genus Croton have potential antibacterial activity. For example, Barbosa [28] reported that the antimicrobial activity of C. urticifolius EOs against strains of S. aureus and E. coli, and Rocha [29] observed that EOs obtained from the leaves of C. tetradenius and C. pulegiodorus inhibited the growth of clinical isolates of S. aureus, leading to cell death.
Other EOs from plants of the genus Croton with a chemical composition similar to that of C. conduplicatus also possess antimicrobial activity against clinically important strains, such as the activity of C. heliotropiifolius [21], C. ferrugineus [30], C. adipatus, C. thurifer and C. collinus [31] essential oils against S. aureus, Klebsiella pneunomiae, Enterococcus faecalis, Candida albicans, Mycobacterium tuberculosis, Escherichia coli and Pseudomonas aeruginosa. The activity of C. cajucara EO against an MRSA strain was related by Azevedo et al. [32], who verified that the EO of this species contained 7-hydroxy-calamenene as the major component, with an MIC value of 4.760 μg mL−1.
The antimicrobial properties are related to the bioactivity of the major compounds as well as to their synergistic action with the minor EO constituents [16,33]. This study presents the first report of the anti-MRSA activity of C. conduplicatus EO.
Studies have associated the antimicrobial action of EOs to the high concentration of 1,8-cineole [15,16], with activity against S. aureus, Escherichia coli, Micrococcus luteus and Bacillus subtilis, among other microorganisms [16,34].
The monoterpene 1,8-cineole, the major component of EOCC, inhibits various microbial strains [35,36,37] including MRSA strains [38]. It shows antibiotic activity both toward the bacteria, such as Escherichia coli, Klebsiella pneumoniae and Pseudomonas aeruginosa, and the biofilms of pathogenic yeasts, such as Candida albicans [39,40,41,42].
The antibacterial activity of 1,8-cineole is associated with oxidative stress and damage to the bacterial cell membrane, causing extravasation of the intracellular contents [36]. Previous studies also reported its synergistic and isolated activity, with a consequent reduction in the MIC of the antibiotic mupirocin and betalactamic antibiotics, such as penicillin, respectively, against MRSA strains [38]. A previous study [3] proved the anti-biofilm and anti-quorum-sensing activity toward MRSA strains, highlighting the importance of combating microbial biofilms to avoid complicated infections and the spread of these strains in hospitals.
Other studies also reported the action of 1,8-cineole, p-cymene, caryophyllene and spathulenol against a broad spectrum of microorganisms, including multidrug-resistant bacteria [17,18,43,44,45]. One of the proposed mechanisms indicates that these compounds permeate the cell wall of bacteria, reversing their resistance and resensitising them to antibiotics [18,45]. A previous study showed the antibacterial activity of EO containing p-cymene against MRSA strains [46]. This compound presents a greater inhibitory potential when associated with other monoterpenes, such as carvacrol and 4-terpineol [47,48]. The p-cymene can affect the membrane integrity of MSSA and MRSA strains, facilitating the passage of other antimicrobial agents and modifying the resistance of these strains to certain antibacterials [49]. Its antibiofilm activity was reported by Miladi et al. [50], who found that p-cymene alone and in combination with tetracycline was effective in preventing biofilm formation in MRSA and MSSA strains as well as clinical S. aureus strains isolated from the human oral cavity.
However, no studies addressed the isolated action of caryophyllene and spathulenol in inhibiting MRSA and MSSA strains. However, EO that contained caryophyllene or spathulenol as one of its major compounds inhibited the action of these strains and their biofilms [51,52,53,54]. This points to the development of studies investigating the potential activity of these compounds in inhibiting MRSA or MSSA strains.
Thus, the synergistic activity of EOCC in combination with betalactamic antibiotics, such as OXA and AMP used in this study, may be related to the joining of the mechanisms of action. The EOCC, containing 1,8-cineole, p-cymene, caryophyllene and spathulenol as major components, causes damage to the cell membrane, with extravasation of intracellular contents, and betalactamic antibiotics act by inhibiting penicillin-binding proteins (PBP), preventing cell wall formation [55]. The combination of the mechanisms of action potentiates both the action of EOCC and those of OXA and AMP against MSSA and MRSA strains.
The MRSA strains have a genetic mutation that results in the production of an alternative PBP, namely PBP2, with a low affinity for penicillin [55], thus ensuring broad resistance to betalactams, except ceftaroline and ceftobiprole [56]. The presence of PBP2 in the cell wall of S. aureus is an example of a specific microbial resistance. Microbial biofilm formation, on the other hand, is a virulence factor that causes nonspecific resistance, especially when prostheses, catheters and other invasive medical devices are infected, in addition to the relationship with endocarditis and osteomyelitis [57].
Given the evidence regarding the bioactivity of the major compounds present in EOCC, it could be inferred that EO from C. conduplicatus presents a potential antimicrobial effect, especially against MRSA strains. This study considerably contributes to the knowledge about plants of the genus Croton, especially regarding the species C. conduplicatus; we confirm the antibacterial activity, synergistic activity against MRSA and MSSA strains and antibiofilm activity of the EO from the leaves of this plant species.
4. Materials and Methods
4.1. Identification and Harvesting of Plant Material
Leaves from C. conduplicatus were collected at Irecê City, Bahia, at −11.345982 S, −41.891216 W, at 14:00 h on 4 February 2021. For the proper botanical identification of the collected plant material, an exsiccate was made and later deposited and registered under number 3217 in the Manoel de Arruda Câmara Herbarium (ACAM) of the State University of Paraiba.
4.2. Obtaining Plant Drug
The leaves from C. conduplicatus were separated from the other aerial parts and then dried in a circulating oven at a temperature of 40 °C for a period of 72 h. The dried leaves were ground in a knife mill to a particle size of approximately 10 mesh, and 704.5 g of the plant drug (PD) was obtained and stored in a hermetically sealed container protected from light.
4.3. Essential Oil Extraction
The EO was obtained through the hydrodistillation technique at 100 °C, using the simple Clevenger apparatus and a heating mantle (Warmnest, UK), for 3 h. The entire PD was used, considering a ratio of 700 mL of distilled water for every 100 g of PD, the amount of distilled water needed to cover it completely. This procedure enabled the extraction of 2.2 mL of C. conduplicatus essential oil (EOCC), as shown in Figure 8. The obtained EOCC was stored under refrigeration.
Figure 8.
Production of the EOCC using the hydrodistillation technique.
4.4. Gas Chromatography Coupled to Mass Spectrometry (GC-MS)
Gas chromatography-mass spectrometry (GC-MS) analysis was conducted on a Clarus 680 gas chromatograph equipped with a PALCOMBI-xt automatic injector, an Elite-5MS column (30 m × 0.25 mm i.d., 0.25 μm) and a Clarus SQ8S mass spectrometer (Perkin Elmer, Waltham, MA, USA). Helium gas was used as a carrier gas at a flow rate of 1 mL/min. The injector was heated to 250 °C with a 1:10 split, and 1.0 μL of the sample was injected. The oven was programmed as follows: 1st step: heating gradient at 35 °C (for 2 min) to 90 °C (for 2 min) at a rate of 10 °C/min; 2nd step: 90 to 130 °C (for 4 min) at a rate of 8 °C/min; 3rd step: 130 to 230 °C (for 2 min) at a rate of 4 °C p/min. The analysis time was 45.50 min. The detector worked in electron ionisation (EI) mode at 70 eV, with an interface temperature of 180 °C (inlet line) and a source (source temp) at 220 °C. Mass fragments were monitored in the range of 40–610 Da. The NIST database from the NIST MS Search Version 2.2 software (National Institute of Standards and Technology, Gaithersburg, MD, USA) was used for identification of the compounds.
The compounds present in the EOCC were identified by comparing their respective mass spectra with those of other previously analysed compounds and with the mass spectra of the NIST database (NIST MS Search Version 2.2), with the retention index (RI) of each compound and by comparing the chemical composition of C. conduplicatus essential oils described in other studies [14,20,21,22]. These analyses were carried out in triplicate.
4.5. Antimicrobial Activity of C. conduplicatus Essential Oil
4.5.1. Microbial Strains and Inoculum Standardisation
The microbial strains selected for this study were obtained from the American Type Culture Collection (ATCC): Methicillin-sensitive Staphylococcus aureus (MSSA) ATCC 25923, methicillin-resistant Staphylococcus aureus (MRSA) ATCC 33591, Escherichia coli ATCC 25922, Pseudomonas aeruginosa ATCC 27853 and Candida albicans ATCC 10231; the strains were kept in the Laboratory of Clinical Analysis of the State University of Paraíba (LAC-UEPB), stored in brain heart infusion broth (BHIB) (DIFCO®) and 20% (v/v) glycerol (LGCBIO).
The inoculum of S. aureus, E. coli and P. aeruginosa strains was standardised according to the document M07 of the Clinical Laboratory Standards Institute-CLSI [58], starting from a 24-h culture in Mueller Hinton broth (MHB) (DIFCO®). The inoculum of C. albicans was standardised according to the CLSI document M27 [59] from a 24-h culture with isolated colonies on potato dextrose agar (PDA). The initial inoculum was standardised to reach a concentration equal to MacFarland’s 0.5 scale in MHB for bacteria and in Sabouraud broth (SB) (DIFCO®) for yeast.
For the antimicrobial screening assay, the initial inocula were diluted to obtain a concentration of 2.0 to 8.0 × 105 CFU/mL for the bacteria and 1.0 to 5.0 × 103 CFU/mL for C. albicans.
4.5.2. Antimicrobial Agents
Oxacillin sodium (OXA) and ampicillin sodium (AMP), obtained from Teuto Laboratory, were used as antimicrobial agents. Polymyxin B sulphate was obtained from Eurofarma and Amphotericin B obtained from Cristalia Laboratory.
For sample preparation, the EOCC was solubilised in a mixture of 3 mL of dimethylsulfoxide (Neon), 2 mL of Tween 80® (Dinâmica) and 6 mL of sterile deionised water. The antimicrobials were solubilised in sterile distilled water. All solutions were filtered through a 0.22-µm membrane before activity testing to ensure sterility.
4.5.3. Antimicrobial Screening
Antimicrobial screening was performed by determining the minimum inhibitory concentration (MIC) and the minimum bactericidal and fungicidal concentration (MBC/FMC).
The MIC of EOCC and antimicrobials was determined by the broth microdilution method as described in the CLSI documents M07 [58] and M27 [59]. For this, serial dilutions of these compounds were performed to obtain final plaque concentrations ranging from 1024 to 0.03 µg/mL. Subsequently, 100 µL of each of these dilutions was added to a 96-well plate and received 100 µL of the standardised inoculum in each well to obtain a final concentration of 1.0 to 4.0 × 105 CFU/mL for the bacterial strains and of 0.5 to 2.5 × 103 CFU/mL for C. albicans.
Growth inhibition was analysed by evaluating growth in broth compared to an untreated growth control by adding a 0.01% resazurin (Sigma-Aldrich®, St. Louis, MO, USA) solution. The MIC was taken as the lowest concentration capable of inhibiting growth after 24 h of incubation at 35 °C. A control well of DMSO/Tween 80®/water diluent (3.0/1.0/6.0) was included to rule out diluent activity. Wells showing the MIC had a sample seeded onto MHB or PDB plates, which were incubated for 24 and 48 h at 35 °C, respectively, and surviving colonies were counted. The MBC or MFC was considered the lowest concentration capable of inhibiting 99.9% of microbial growth after the incubation period.
4.5.4. Checkerboard Assay against S. aureus
Dilutions of EOCC, oxacillin (OXA) and ampicillin (AMP) were performed in MHB. From these dilutions, 50-µL aliquots were added into 96-well microplates to obtain a final concentration equal to eight dilutions lower than the MIC of EOCC and nine dilutions lower than that of OXA and AMP. Then, 100 µL of the standardised suspension of the MSSA and MRSA strains (105 CFU/well) was added to each well. The plates were incubated for 24 h at 35 °C, and growth inhibition was assessed by comparison with the growth control group. The data were interpreted after calculating the Fractional Inhibition Concentration (FICi) values using the following equation:
| FICi = MICEOCC+AA/MICEOCC + MICEOCC+AA/MICAA | (1) |
where:
MICEOCC: MIC of the essential oil from C. conduplicatus;
MICAA: MIC of the antimicrobial agent;
MICEOCC+AA: MIC of EOCC in combination with antimicrobial agents.
The combination was considered synergistic when FICi ≤ 0.5, additive when 0.5 < FICi ≤ 1, indifferent when 1 < FICi ≤ 2, antagonistic when FICi ≥ 2 [60]. Tests were performed in a triplicate of independent experiments.
4.5.5. Activity against S. aureus Biofilm Formation
To evaluate the activity of EOCC and its combinations with antifungals at subinhibitory concentrations on the formation of biofilms of S. aureus, the methodology described by Manoharan et al. [61], with modifications, was used. Starting from 24-h cultures of MSSA and MRSA strains in MHB, a 50-µL aliquot was inoculated into MHB and then incubated at 35 °C until turbidity equivalent to 0.5 McFarland scale (1.0 × 108 to 2.0 × 108 CFU/mL) was reached. From this culture, a 100-µL aliquot was dispensed into the wells of the microdilution plates. Subsequently, 100 µL of the compounds and their combinations was dispensed at final plate concentrations corresponding to the MIC and the FICi determined via the checkerboard method, and the plates were incubated at 35 ± 2 °C for 24 h. A positive control of biofilm formation was included, containing 100 µL MHB and 100 µL of the inoculum, and the negative control consisted of 200 µL MHB.
The biomass of the biofilm formed after the treatments was determined according to Munusamy, Vadivelu and Tay [23] via staining with crystal violet, with modifications.
After the incubation period, the plates were washed three times with sterile saline solution (NaCl 0.85%) to remove any cells that were not adhered to the biofilm and placed in a drying oven at 40 °C for 20 min. Subsequently, 200 µL of a 0.4% crystal violet solution was added to all wells, and the plates were incubated for 45 min. After incubation, the plates were washed with sterile distilled water and oven-dried at 40 °C for 20 min. After drying the wells, 200 µL of 96% ethyl alcohol was added for 45 min to detain the biofilm.
To perform the reading, 100 µL was removed and placed in a new 96-well flat-bottom plate. Reading was performed by optical density (OD) in a microplate reader (xMark™, Bio-Rad, Hercules, CA, USA) at a wavelength of 585 nm. The results were expressed as the percentage of inhibition of biofilm formation compared to the positive control, which represents 100% of biofilm formation.
4.5.6. Activity against S. aureus Formed Biofilm
Starting from a 24-h culture of S. aureus strains in MHB, a 200-µL aliquot was inoculated into 20 mL of MHB and incubated at 35 °C until turbidity equivalent to McFarland’s 0.5 scale (1.0 × 106 to 5.0 × 106 CFU/mL) was reached. From this culture, 200 µL was dispensed into all 96 wells of the microdilution plates and incubated at 35 ± 2 °C for 24 h to form the biofilm. After the incubation period, the culture medium was carefully removed, and the wells were aseptically washed three times with sterile saline solution (NaCl) at 0.85% to remove the cells that were not adhered to the wells; subsequently, the plates were sealed and placed on a flat surface for 20 min at room temperature (25 °C) for drying. The dried biofilms were spiked with 200 µL of the compounds and their combinations, and the plates were re-incubated at 35 ± 2 °C for 24 h. A positive control for biofilm formation was included, consisting of 100 µL of MHB and 100 µL of the inoculum, and the negative control consisted of 200 µL of MHB.
To evaluate the activity of EOCC and its combinations with OXA and AMP on biofilms formed by S. aureus strains, the methodology described by Uppuluri et al. [62] was used, with modifications.
After the incubation period, the culture medium was removed, and the plates were washed three times with sterile 0.85% NaCl (Dinâmica) to remove any non-adhered cells; subsequently, the plates were placed in an oven at 40 °C for 20 min for drying. The biofilm biomass was determined according to Munusamy, Vadivelu and Tay [23], with some modifications, as described above. The results were expressed as percentage of inhibition of the biofilm formed compared to the positive control (100% of biofilm formation), indicating action against the biofilm formed by S. aureus.
4.5.7. Statistical Analysis
The experiments were performed in triplicates of independent tests, and results were expressed as the mean and standard deviation of the percentage of biofilm inhibition, calculated in the Office Excel 2019 software. The results were submitted to statistical analysis by applying the t-test, performing analysis of variance and evaluating the statistical difference among the treatments. Statistical significance was set at p < 0.05.
5. Conclusions
The EOCC showed antibacterial activity against methicillin-susceptible and methicillin-resistant Staphylococcus aureus strains. This effect was observed together with a potential synergistic effect when EOCC was associated with OXA and AMP, to the point of modifying the resistance profile of MSSA and MRSA strains. Furthermore, EOCC also showed a potential effect of inhibiting biofilm formation and reducing mature biofilms of MRSA and MSSA strains. These results were associated with the chemical composition of EOCC, which showed 1,8-cineole, p-cymene, caryophyllene and spathulenol as the main constituents, compounds known for their activity against multidrug-resistant bacterial strains. These results provide a solid reference for further studies on EOCC in combination with different antibiotics to evaluate its mechanism of action and propose pharmaceutical formulations, with the aim to broaden the therapeutic resources against infections caused by pathogenic microorganisms.
Acknowledgments
Authors are grateful to Marcus Vinicius Lia Fook (Laboratory of Evaluation and Development of Biomaterials-CERTBIO) for providing GC-MS analysis, Baldoíno Sonildo da Nóbrega (Federal Institute of Paraíba-IFPB) for providing the statistical analysis of anti-biofilm activity and Antônio Carlos Santos Rocha Júnior for the collection of the botanical material. We also thank the Pharmaceuticals and Medicines Research Institute of the Federal University of Paraiba (IPeFarM/UFPB), the Higher Education Personnel Improvement Coordination (CAPES-Brazil) (Process No. 88887.505803/2020-00), National Council for Scientific and Technological Development (CNPq-Brazil) and Paraiba State Research Foundation (FAPESQ) for financial support.
Author Contributions
Conceptualization, data curation, investigation, methodology, writing-original draft, extraction and treatment of plant material and essential oil, G.D.d.O.; Data curation, investigation, acquisition, visualization, interpretation of antimicrobial data, writing-original draft and maintenance of microbial strains, W.R.V.d.R.; Data curation, investigation, acquisition, visualization, analysis and interpretation of GC-MS data, J.F.B.R.; Project administration, resources, supervision, conceptualization and writing and review, H.d.S.A. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
State University of Paraiba/UEPB, grant No. 003/2022. This study was financed in part by FAPESQ, Funding nº 04/2022—Support for the Scientific and Technological Development of the State of Paraíba—grant #3783/2022-0.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Cheung G.Y.C., Bae J.S., Otto M. Pathogenicity and virulence of Staphylococcus aureus. Virulence. 2021;12:547–569. doi: 10.1080/21505594.2021.1878688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mlynarczyk-Bonikowska B., Kowalewski C., Krolak-Ulinska A., Marusza W. Molecular Mechanisms of Drug Resistance in Staphylococcus aureus. Int. J. Mol. Sci. 2022;23:88. doi: 10.3390/ijms23158088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Merghni A., Noumi E., Hadded O., Dridi N., Panwar H., Ceylan O., Mastouri M., Snoussi M. Assessment of the antibiofilm and antiquorum sensing activities of Eucalyptus globulus essential oil and its main component 1,8-cineole against methicillin-resistant Staphylococcus aureus strains. Microb. Pathog. 2018;118:74–80. doi: 10.1016/j.micpath.2018.03.006. [DOI] [PubMed] [Google Scholar]
- 4.Flemming H.-C., Wingender J. The biofilm matrix. Nat. Rev. Microbiol. 2010;8:623–633. doi: 10.1038/nrmicro2415. [DOI] [PubMed] [Google Scholar]
- 5.McConoughey S.J., Howlin R., Granger J.F., Manring M.M., Calhoun J.H., Shirtliff M., Kathju S., Stoodley P. Biofilms in periprosthetic orthopedic infections. Future Microbiol. 2014;9:987–1007. doi: 10.2217/fmb.14.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.World Health Organization . List of Bacteria for Which New Antibiotics Are Urgently Needed. World Health Organization; Geneva, Switzerland: 2017. [Google Scholar]
- 7.Aminov R. History of antimicrobial drug discovery: Major classes and health impact. Biochem. Pharmacol. 2017;133:4–19. doi: 10.1016/j.bcp.2016.10.001. [DOI] [PubMed] [Google Scholar]
- 8.Shin J., Prabhakaran V.-S., Kim K.-s. The multi-faceted potential of plant-derived metabolites as antimicrobial agents against multidrug-resistant pathogens. Microb. Pathog. 2018;116:209–214. doi: 10.1016/j.micpath.2018.01.043. [DOI] [PubMed] [Google Scholar]
- 9.Mérillon J.-M., Riviere C. Natural Antimicrobial Agents. Vol. 19 Springer; Berlin/Heidelberg, Germany: 2018. [Google Scholar]
- 10.Sreepian A., Popruk S., Nutalai D., Phutthanu C., Sreepian P.M. Antibacterial Activities and Synergistic Interaction of Citrus Essential Oils and Limonene with Gentamicin against Clinically Isolated Methicillin-Resistant Staphylococcus aureus. Sci. World J. 2022;2022:1–12. doi: 10.1155/2022/8418287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coimbra A., Miguel S., Ribeiro M., Coutinho P., Silva L., Duarte A.P., Ferreira S. Thymus zygis Essential Oil: Phytochemical Characterization, Bioactivity Evaluation and Synergistic Effect with Antibiotics against Staphylococcus aureus. Antibiotics. 2022;11:146. doi: 10.3390/antibiotics11020146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Salatino A., Salatino M.L.F., Negri G. Traditional uses, chemistry and pharmacology of Croton species (Euphorbiaceae) J. Braz. Chem. Soc. 2007;18:11–33. doi: 10.1590/S0103-50532007000100002. [DOI] [Google Scholar]
- 13.Cartaxo S.L., de Almeida Souza M.M., de Albuquerque U.P. Medicinal plants with bioprospecting potential used in semi-arid northeastern Brazil. J. Ethnopharmacol. 2010;131:326–342. doi: 10.1016/j.jep.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 14.Oliveira Júnior R.G., Ferraz C.A.A., Silva J.C., de Andrade Teles R.B., Silva M.G., Diniz T.C., Dos Santos U.S., de Souza A.V.V., Nunes C.E.P., Salvador M.J., et al. Neuropharmacological effects of essential oil from the leaves of Croton conduplicatus Kunth and possible mechanisms of action involved. J. Ethnopharmacol. 2018;221:65–76. doi: 10.1016/j.jep.2018.04.009. [DOI] [PubMed] [Google Scholar]
- 15.Zhou S., Wei C., Zhang C., Han C., Kuchkarova N., Shao H. Chemical Composition, Phytotoxic, Antimicrobial and Insecticidal Activity of the Essential Oils of Dracocephalum integrifolium. Toxins. 2019;11:598. doi: 10.3390/toxins11100598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nafis A., Kasrati A., Jamali C.A., Custódio L., Vitalini S., Iriti M., Hassani L. A Comparative Study of the in Vitro Antimicrobial and Synergistic Effect of Essential Oils from Laurus nobilis L. and Prunus armeniaca L. from Morocco with Antimicrobial Drugs: New Approach for Health Promoting Products. Antibiotics. 2020;9:140. doi: 10.3390/antibiotics9040140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sa C., Liu J., Dong Y., Jiang L., Gentana G., Wurita A. Quantification of eucalyptol (1,8-cineole) in rat serum by gas chromatography-mass/mass spectrometry and its application to a rat pharmacokinetic study. Biomed. Chromatogr. 2021;35:1–10. doi: 10.1002/bmc.5080. [DOI] [PubMed] [Google Scholar]
- 18.Agreles M.A.A., Cavalcanti I.D.L., Cavalcanti I.M.F. The Role of Essential Oils in the Inhibition of Efflux Pumps and Reversion of Bacterial Resistance to Antimicrobials. Curr. Microbiol. 2021;78:3609–3619. doi: 10.1007/s00284-021-02635-1. [DOI] [PubMed] [Google Scholar]
- 19.Adams R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry. Vol. 456 Allured publishing corporation Carol Stream; Carol Stream, IL, USA: 2007. [Google Scholar]
- 20.De Oliveira Júnior R.G., Ferraz C.A.A., Silva J.C., De Oliveira A.P., Diniz T.C., E Silva M.G., Quintans Júnior L.J., De Souza A.V.V., Dos Santos U.S., Turatti I.C.C., et al. Antinociceptive Effect of the Essential Oil from Croton conduplicatus Kunth (Euphorbiaceae) Molecules. 2017;22:900. doi: 10.3390/molecules22060900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Almeida J., Souza A.V., Oliveira A.P., Santos U., Souza M., Bispo L., Turatti Z.C., Lopes N. Chemical Composition of Essential Oils from Croton conduplicatus (Euphorbiaceae) in Two Different Seasons. J. Essent. Oil-Bear. Plants. 2014;17:1137–1145. doi: 10.1080/0972060X.2014.931254. [DOI] [Google Scholar]
- 22.Castro K.N.d.C., Chagas A.C.d.S., Costa-Júnior L.M., Canuto K.M., Brito E.S.d., Rodrigues T.H.S., de Andrade I.M. Acaricidal potential of volatile oils from Croton species on Rhipicephalus microplus. Rev. Bras. Farmacogn. 2020;29:811–815. doi: 10.1016/j.bjp.2019.09.001. [DOI] [Google Scholar]
- 23.Munusamy K., Vadivelu J., Tay S.T. A study on Candida biofilm growth characteristics and its susceptibility to aureobasidin A. Rev. Iberoam. Micol. 2018;35:68–72. doi: 10.1016/j.riam.2017.07.001. [DOI] [PubMed] [Google Scholar]
- 24.Oliveira A.C., Costa-Lima T.C., Souza A.V.V., Gonçalves-Gervásio R.d.C.R. Essential oils activity from plants of the Brazilian Caatinga on the vegetable leafminer. Pesqui. Agropecu. Trop. 2020;50:1–8. doi: 10.1590/1983-40632020v5058313. [DOI] [Google Scholar]
- 25.de Araújo L.G., Veras Neto J.G., de Oliveira Alves J.V., de Veras B.O., Vanusa da Silva M., Bacalhau Rodrigues J.F., Fook M.V.L., Amoah S.K.S., Menezes Torres M.d.C. Chemodiversity and antibacterial activity of the essential oil of leaves of Croton argyrophyllus Kunth. Chem. Biodivers. 2020;17:1–9. doi: 10.1002/cbdv.202000575. [DOI] [PubMed] [Google Scholar]
- 26.Clinical and Laboratory Standards Institute . M100-Performance Standards for Antimicrobial Susceptibility Testing. Clinical and Laboratory Standards Institute; Wayne, IL, USA: 2017. p. 249. [Google Scholar]
- 27.Bakkali F., Averbeck S., Averbeck D., Idaomar M. Biological effects of essential oils—A review. Food Chem. Toxicol. 2008;46:446–475. doi: 10.1016/j.fct.2007.09.106. [DOI] [PubMed] [Google Scholar]
- 28.Barbosa A.J.C. Dissertation. State University of Paraiba; 2021. [(accessed on 13 December 2022)]. Composição química e atividade antibacteriana dos óleos essenciais de Croton urticifolius LAM. e Croton adamantinus MÜLL. ARG. (EUPHORBIACEAE) Available online: https://repositorio.ufpe.br/handle/123456789/25510. [Google Scholar]
- 29.Rocha R.R. Dissertation. Federal University of Ceará; 2020. [(accessed on 13 December 2022)]. Estudo comparativo sobre a composição química, atividade antibacteriana e efeito sinérgico dos óleos essenciais de Croton tetradenius Baill. e c. pulegiodorus Baill. Contra isolados de Staphylococcus aureus. Available online: https://repositorio.ufpb.br/jspui/handle/tede/4016. [Google Scholar]
- 30.Valarezo E., Gaona-Granda G., Morocho V., Cartuche L., Calva J., Meneses M.A. Chemical Constituents of the Essential Oil from Ecuadorian Endemic Species Croton ferrugineus and Its Antimicrobial, Antioxidant and α-Glucosidase Inhibitory Activity. Molecules. 2021;26:4608. doi: 10.3390/molecules26154608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cucho-Medrano J.L.L., Mendoza-Beingolea S.W., Fuertes-Ruitón C.M., Salazar-Salvatierra M.E., Herrera-Calderon O. Chemical Profile of the Volatile Constituents and Antimicrobial Activity of the Essential Oils from Croton adipatus, Croton thurifer, and Croton collinus. Antibiotics. 2021;10:1387. doi: 10.3390/antibiotics10111387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Azevedo M.M.B., Chaves F.C.M., Almeida C.A., Bizzo H.R., Duarte R.S., Campos-Takaki G.M., Alviano C.S., Alviano D.S. Antioxidant and Antimicrobial Activities of 7-Hydroxy-calamenene-Rich Essential Oils from Croton cajucara Benth. Molecules. 2013;18:1128–1137. doi: 10.3390/molecules18011128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhao Q., Luan X., Zheng M., Tian X.-H., Zhao J., Zhang W.-D., Ma B.-L. Synergistic mechanisms of constituents in herbal extracts during intestinal absorption: Focus on natural occurring nanoparticles. Pharmaceutics. 2020;12:128. doi: 10.3390/pharmaceutics12020128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nafis A., Elhidar N., Oubaha B., Samri S.E., Niedermeyer T., Ouhdouch Y., Hassani L., Barakate M. Screening for Non-polyenic Antifungal Produced by Actinobacteria from Moroccan Habitats: Assessment of Antimycin A19 Production by Streptomyces albidoflavus AS25. Int. J. Mol. Cell. Med. 2018;7:133–145. doi: 10.22088/IJMCM.BUMS.7.2.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ivanov M., Kannan A., Stojković D.S., Glamočlija J., Calhelha R.C., Ferreira I., Sanglard D., Soković M. Camphor and Eucalyptol-Anticandidal Spectrum, Antivirulence Effect, Efflux Pumps Interference and Cytotoxicity. Int. J. Mol. Sci. 2021;22:483. doi: 10.3390/ijms22020483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moo C.-L., Osman M.A., Yang S.-K., Yap W.-S., Ismail S., Lim S.-H.-E., Chong C.-M., Lai K.-S. Antimicrobial activity and mode of action of 1,8-cineol against carbapenemase-producing Klebsiella pneumoniae. Sci. Rep. 2021;11:1–13. doi: 10.1038/s41598-021-00249-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cai Z.M., Peng J.Q., Chen Y., Tao L., Zhang Y.Y., Fu L.Y., Long Q.D., Shen X.C. 1,8-Cineole: A review of source, biological activities, and application. J. Asian Nat. Prod. Res. 2021;23:938–954. doi: 10.1080/10286020.2020.1839432. [DOI] [PubMed] [Google Scholar]
- 38.Kwiatkowski P., Pruss A., Wojciuk B., Dołęgowska B., Wajs-Bonikowska A., Sienkiewicz M., Mężyńska M., Łopusiewicz Ł. The Influence of Essential Oil Compounds on Antibacterial Activity of Mupirocin-Susceptible and Induced Low-Level Mupirocin-Resistant MRSA Strains. Molecules. 2019;24:3105. doi: 10.3390/molecules24173105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vijayakumar K., Manigandan V., Jeyapragash D., Bharathidasan V., Anandharaj B., Sathya M. Eucalyptol inhibits biofilm formation of Streptococcus pyogenes and its mediated virulence factors. J. Med. Microbiol. 2020;69:1308–1318. doi: 10.1099/jmm.0.001253. [DOI] [PubMed] [Google Scholar]
- 40.Keymaram M., Falahati M., Farahyar S., Lotfali E., Abolghasemi S., Mahmoudi S., Sadeghi F., Khalandi H., Ghasemi R., Shamsaei S., et al. Anti-biofilm properties of eucalyptol in combination with antifungals against Candida albicans isolates in patients with hematological malignancy. Arch. Microbiol. 2022;204:1–8. doi: 10.1007/s00203-022-02911-z. [DOI] [PubMed] [Google Scholar]
- 41.Vazquez N.M., Mariani F., Torres P.S., Moreno S., Galvan E.M. Cell death and biomass reduction in biofilms of multidrug resistant extended spectrum β-lactamase-producing uropathogenic Escherichia coli isolates by 1, 8-cineole. PLoS ONE. 2020;15:e0241978. doi: 10.1371/journal.pone.0241978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Karuppiah V., Thirunanasambandham R., Thangaraj G. Anti-quorum sensing and antibiofilm potential of 1,8-cineole derived from Musa paradisiaca against Pseudomonas aeruginosa strain PAO1. World J. Microbiol. Biotechnol. 2021;37:1–12. doi: 10.1007/s11274-021-03029-y. [DOI] [PubMed] [Google Scholar]
- 43.Badalamenti N., Bruno M., Gagliano Candela R., Maggi F. Chemical composition of the essential oil of Elaeoselinum asclepium (L.) Bertol subsp. meoides (Desf.) Fiori (Umbelliferae) collected wild in Central Sicily and its antimicrobial activity. Nat. Prod. Res. 2022;36:789–797. doi: 10.1080/14786419.2020.1805607. [DOI] [PubMed] [Google Scholar]
- 44.Vasconcelos A.A., Veras I.N.d.S., Vasconcelos M.A.d., Andrade A.L., dos Santos H.S., Bandeira P.N., Souza E.B.d., Albuquerque M.R.J.R., Teixeira E.H. Chemical composition determination and evaluation of the antibacterial activity of essential oils from Ruellia asperula (Mart. Ex Ness) Lindau and Ruellia paniculata L. against oral Streptococci. Nat. Prod. Res. 2021:1–5. doi: 10.1080/14786419.2021.1960521. [DOI] [PubMed] [Google Scholar]
- 45.Mączka W., Duda-Madej A., Górny A., Grabarczyk M., Wińska K. Can Eucalyptol Replace Antibiotics? Molecules. 2021;26:4933. doi: 10.3390/molecules26164933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li H., Yang T., Li F.Y., Yao Y., Sun Z.M. Antibacterial activity and mechanism of action of Monarda punctata essential oil and its main components against common bacterial pathogens in respiratory tract. Int J. Clin. Exp. Pathol. 2014;7:7389–7398. [PMC free article] [PubMed] [Google Scholar]
- 47.Soković M., Glamočlija J., Marin P.D., Brkić D., Griensven L.J.L.D.v. Antibacterial Effects of the Essential Oils of Commonly Consumed Medicinal Herbs Using an In Vitro Model. Molecules. 2010;15:7532–7546. doi: 10.3390/molecules15117532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Marchese A., Arciola C.R., Barbieri R., Silva A.S., Nabavi S.F., Tsetegho Sokeng A.J., Izadi M., Jafari N.J., Suntar I., Daglia M., et al. Update on Monoterpenes as Antimicrobial Agents: A Particular Focus on p-Cymene. Materials. 2017;10:947. doi: 10.3390/ma10080947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mouwakeh A., Kincses A., Nové M., Mosolygó T., Mohácsi-Farkas C., Kiskó G., Spengler G. Nigella sativa essential oil and its bioactive compounds as resistance modifiers against Staphylococcus aureus. Phytother. Res. 2019;33:1010–1018. doi: 10.1002/ptr.6294. [DOI] [PubMed] [Google Scholar]
- 50.Miladi H., Zmantar T., Kouidhi B., Al Qurashi Y.M.A., Bakhrouf A., Chaabouni Y., Mahdouani K., Chaieb K. Synergistic effect of eugenol, carvacrol, thymol, p-cymene and γ-terpinene on inhibition of drug resistance and biofilm formation of oral bacteria. Microb. Pathog. 2017;112:156–163. doi: 10.1016/j.micpath.2017.09.057. [DOI] [PubMed] [Google Scholar]
- 51.Wu J.-G., Peng W., Yi J., Wu Y.-B., Chen T.-Q., Wong K.-H., Wu J.-Z. Chemical composition, antimicrobial activity against Staphylococcus aureus and a pro-apoptotic effect in SGC-7901 of the essential oil from Toona sinensis (A. Juss.) Roem. leaves. J. Ethnopharmacol. 2014;154:198–205. doi: 10.1016/j.jep.2014.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Salinas C., Florentín G., Rodríguez F., Alvarenga N., Guillén R. Terpenes Combinations Inhibit Biofilm Formation in Staphyloccocus aureus by Interfering with Initial Adhesion. Microorganisms. 2022;10:1527. doi: 10.3390/microorganisms10081527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tadić V., Oliva A., Božović M., Cipolla A., De Angelis M., Vullo V., Garzoli S., Ragno R. Chemical and Antimicrobial Analyses of Sideritis romana L. subsp. purpurea (Tal. ex Benth.) Heywood, an Endemic of the Western Balkan. Molecules. 2017;22:1395. doi: 10.3390/molecules22091395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Aziz P., Muhammad N., Intisar A., Abid M.A., Din M.I., Yaseen M., Kousar R., Aamir A., Quratulain , Ejaz R. Constituents and antibacterial activity of leaf essential oil of Plectranthus scutellarioides. Plant Biosyst. 2021;155:1247–1252. doi: 10.1080/11263504.2020.1837279. [DOI] [Google Scholar]
- 55.Reynolds P.E., Brown D.F. Penicillin-binding proteins of beta-lactam-resistant strains of Staphylococcus aureus. Effect of growth conditions. FEBS Lett. 1985;192:28–32. doi: 10.1016/0014-5793(85)80036-3. [DOI] [PubMed] [Google Scholar]
- 56.Chambers H.F., Deleo F.R. Waves of resistance: Staphylococcus aureus in the antibiotic era. Nat. Rev. Microbiol. 2009;7:629–641. doi: 10.1038/nrmicro2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Otto M. Staphylococcal biofilms. Curr. Top. Microbiol. Immunol. 2008;322:207–228. doi: 10.1007/978-3-540-75418-3_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.(CLSI), C.L.S.I. M07-Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically. 2018. [(accessed on 13 December 2022)]. p. 91. Available online: https://clsi.org/media/1928/m07ed11_sample.pdf.
- 59.(CLSI), C.L.S.I. M27-Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts. 2017, 4th Edition, 34. [(accessed on 13 December 2022)]. Available online: https://clsi.org/media/1897/m27ed4_sample.pdf.
- 60.Lorian V. Antibiotics in Laboratory Medicine. Lippincott Williams & Wilkins; Philadelphia, PA, USA: 2005. [Google Scholar]
- 61.Manoharan R.K., Lee J.-H., Kim Y.-G., Kim S.-I., Lee J. Inhibitory effects of the essential oils α-longipinene and linalool on biofilm formation and hyphal growth of Candida albicans. Biofouling. 2017;33:143–155. doi: 10.1080/08927014.2017.1280731. [DOI] [PubMed] [Google Scholar]
- 62.Uppuluri P., Pierce C.G., López-Ribot J.L. Candida albicans biofilm formation and its clinical consequences. Future Microbiol. 2009;4:1235–1237. doi: 10.2217/fmb.09.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are contained within the article.