Abstract
In frontotemporal dementia (FTD), left-lateralized atrophy patterns have been associated with elevations in certain positive emotions. Here, we investigated whether positive emotional reactivity is enhanced in semantic variant primary progressive aphasia (svPPA), an FTD syndrome that targets the left anterior temporal lobe. Sixty-one participants (16 people with svPPA, 24 people with behavioral variant FTD, and 21 healthy controls) viewed six 90-second trials that were comprised of a series of photographs; each trial was designed to elicit a specific positive emotion, negative emotion, or no emotion. Participants rated their positive emotional experience after each trial, and their smiling behavior was coded with the Facial Action Coding System. Results indicated that positive emotional experience and smiling were elevated in svPPA in response to numerous affective and non-affective stimuli. Voxel-based morphometry analyses revealed that greater positive emotional experience and greater smiling in the patients were both associated with smaller gray matter volume in the left superior temporal gyrus (pFWE<.05), among other left-lateralized frontotemporal regions. Whereas enhanced positive emotional experience related to atrophy in middle superior temporal gyrus and structures that promote cognitive control and emotion regulation, heightened smiling related to atrophy in posterior superior temporal gyrus and structures that support motor control. Our results suggest positive emotional reactivity is elevated in svPPA and offer new evidence that atrophy of left-lateralized emotion-relevant systems relates to enhanced positive emotions in FTD.
Keywords: euphoria, elation, semantic dementia, mood, neurodegeneration
1. Introduction
Positive emotions play a central role in human relationships. Amusement, nurturant love, compassion, and awe, among others, make up a family of positive emotions that helps us to forge, solidify, and maintain lasting social bonds (Cavanaugh et al., 2015; Fredrickson, 1998, 2013; Shiota et al., 2011). Although positive emotions often foster kindness, generosity, and trust, they can be problematic when they are context-incongruent, too elevated, or too sustained (Gilbert et al., 2013; Gruber, 2011; Gruber & Purcell, 2015). Dysregulated positive emotions are a core feature of some clinical disorders including bipolar disorder, for example, which is characterized by mania—intermittent periods of heightened positive emotional experience or mixed feelings of euphoria and irritability. During manic episodes, individuals with bipolar disorder may exhibit increased risk-taking, flight of ideas, and overfamiliarity, approach-related behaviors that can lead to deleterious outcomes (Gilbert et al., 2013; Hechtman et al., 2013). Thus, while phasic bursts of positive emotions may confer interpersonal advantages, dysregulated positive emotions may interfere with social functioning.
Frontotemporal dementia (FTD) is a neurodegenerative disorder that also affects positive emotions. In FTD, there is progressive deterioration of the frontal and anterior temporal lobes, and, prior to diagnosis, many individuals are diagnosed with psychiatric illnesses, such as bipolar disorder, because of overlapping clinical features including elevated mood (Mendez et al., 2006; Woolley et al., 2011). Behavioral variant FTD (bvFTD) is a clinical subtype of FTD characterized by apathy (Snowden et al., 2001), disinhibition, and decreased empathy (Rankin et al., 2006), among other symptoms (Rascovsky et al., 2011). Bilateral or right-lateralized atrophy is thought to characterize most cases of bvFTD (Seeley et al., 2008), which typically targets the ventral anterior insula as well as the anterior cingulate cortex, amygdala, hypothalamus, and brainstem (Brown et al., 2019; Seeley et al., 2009). Many people with bvFTD exhibit diminished emotional reactivity in response to negative affective stimuli such as those that elicit embarrassment (Sturm et al., 2008), disgust (Eckart et al., 2012; Muhtadie et al., 2019), and sadness (Hua et al., 2020). While some individuals with bvFTD exhibit lower positive emotions and social connectedness (Pressman et al., 2017; Takeda et al., 2019), others become more sensitive to stimuli that are rewarding or amusing (Clark et al., 2015; Perry et al., 2014; Sturm et al., 2015). In some cases, people with bvFTD may even exhibit heightened positive emotional reactions to stimuli that typically elicit a negative response (e.g., photographs of negative and neutral faces), suggesting impaired downregulation of positive emotions in various contexts (Hua et al., 2018).
Semantic variant primary progressive aphasia (svPPA) is a clinical subtype of FTD characterized by loss of semantic knowledge (Gorno-Tempini et al., 2011; Lambon Ralph et al., 2010). Although svPPA often begins with focal anterior temporal lobe dysfunction in the left hemisphere, atrophy soon spreads from the left to the right anterior temporal lobe and on to the ventral anterior insula and orbitofrontal cortex (Collins et al., 2017; Guo et al., 2013; Landin-Romero et al., 2016; O’Connor et al., 2016; Seeley et al., 2005). Changes in social cognition, empathy, and emotions also occur in svPPA (Binney et al., 2016; Borghesani et al., 2019; Kamath et al., 2018; Thompson et al., 2003) but have received relatively less attention to date (Kumfor & Piguet, 2012). Prior studies have found that people with svPPA have impaired emotion recognition (Fittipaldi et al., 2019; Irish et al., 2013; Lindquist et al., 2014; Macoir et al., 2019; Rosen et al., 2002) and perspective-taking (Irish et al., 2014; Shdo et al., 2016). Despite these deficits, patients may exhibit elevated mood (Shimizu et al., 2011), enhanced social interest (Mendez et al., 2006; Snowden et al., 2001; Sturm et al., 2011), and heightened positive emotional facial expressions (Kumfor et al., 2019), which suggests certain positive emotions may be elevated in svPPA.
Our previous work has suggested that elevated positive emotional reactivity in FTD is associated with left-lateralized frontotemporal atrophy patterns (Sturm et al., 2015). Across FTD syndromes, smaller gray matter volume in left dorsal anterior insula and left ventrolateral prefrontal cortex, regions that support emotion regulation and behavioral inhibition (Aron et al., 2004; Ochsner et al., 2009b), was associated with higher levels of positive emotional reactivity (i.e., smiling and laughing behavior as well as autonomic nervous system reactivity). These results suggested that atrophy in emotion regulation systems centered in the left hemisphere may “release” emotion generators (Saper, 2002) and thereby accentuate positive emotional reactivity. The goal of the present study was to build on this work by measuring positive emotional reactivity in svPPA, a disorder that predominantly targets the left anterior temporal lobe and adjacent frontotemporal regions, and to compare this group to healthy controls and to patients with bvFTD. We expected that atrophy in left-lateralized emotion regulation systems (e.g., dorsal anterior insula and ventrolateral prefrontal cortex, in particular) would relate to heightened positive emotional reactivity in svPPA across contexts—not only in response to positive stimuli but also in response to neutral and negative stimuli—and that people with svPPA would show greater positive emotional reactivity than healthy controls and those with bvFTD.
2. Material and Methods
2.1. Participants
Sixty-one participants were included in the present study: 16 people with svPPA (Gorno-Tempini et al., 2011), 24 people with bvFTD (Rascovsky et al., 2011), and 21 healthy older control participants. No individuals with bvFTD had comorbid motor neuron disease. Two individuals with bvFTD were initially included in a larger sample but were excluded from all analyses due to a lack of attention during the task. Diagnoses were made by a multidisciplinary team and based on thorough neurological, behavioral, neuropsychological, and neuroimaging evaluations. Patients were recruited to the study through referrals from the University of California, San Francisco (UCSF) Memory and Aging Center or from external clinics. Healthy older control participants were recruited from the community through local advertisements. The healthy controls underwent an identical diagnostic evaluation as the patients and were included if they had an unremarkable neurological exam, a normal magnetic resonance imaging (MRI) scan, and no functional or cognitive deficits. The Clinical Dementia Rating scale (CDR), a measure of functional impairment, was completed with informants (Morris, 1993, 1997), and we used the CDR Total and CDR – Sum of Boxes (CDR-SOB) scores to quantify disease severity. Information about participants’ psychiatric medication use (i.e., medications prescribed for depression, anxiety, and psychosis) was collected at the time of testing. Demographic, cognitive, and functional characteristics of all participants are presented in Table 1. The study procedures were approved by the UCSF Committee on Human Research. All participants, or their surrogates, provided informed consent. No part of the study procedures was pre-registered prior to the research being conducted. Inclusion and exclusion criteria were established prior to data analysis. The sample size was informed by previous research of this type in these clinical syndromes (Kumfor et al., 2019).
Table 1.
Participant demographics and clinical data.
| Characteristics | Healthy Controls | bvFTD | svPPA | Test Statistic | p-value |
|---|---|---|---|---|---|
|
| |||||
| n | 21 | 24 | 16 | ||
| Age | 70.1 (6.3) | 65.2 (8.3) | 64.7 (6.2) | F(2,58)=3.57 | .03 |
| Sex (Male/Female) | 9/12 | 15/9 | 6/10 | χ2(2, N=61)=2.91 | .23 |
| Education | 16.3 (3.5) | 16.3 (3.5) | 17.9 (3.1) | F(2,56)=2.18 | .13 |
| CDR Total Score (0–3) | 0.0 (0.1) | 1.3 (0.6) | 0.8 (0.4) | F(1,38)=11.01 | <.01 |
| CDR-SOB (0–12) | 0.1 (0.3) | 7.2 (2.7) | 4.2 (2.2) | F(1,38)=13.89 | <.001 |
| MMSE (0–30) | 29.1 (1.2) | 25.3 (3.3) | 23.3 (4.7) | F(1,38)=2.49 | .12 |
| PPVT (0–16) | 16.0 (0) | 14.5 (1.7) | 10.6 (2.6) | F(1,33)=30.07 | <.001 |
| BNT (0–15) | 14.8 (0.5) | 12.8 (2.9) | 5.1 (3.2) | F(1,37)=64.45 | <.001 |
| Psychiatric medications (Y/N) | 2/19 | 18/6 | 11/5 | χ2(1, N=40)=.19 | .66 |
Statistical comparisons were limited to the bvFTD and svPPA groups for the clinical measures (i.e., CDR Total Score, CDR-SOB, MMSE, PPVT, BNT, and psychiatric medications) due to heterogeneity of variance in the healthy control group. bvFTD=behavioral variant frontotemporal dementia, svPPA=semantic variant primary progressive aphasia, MMSE=Mini-Mental State Examination, CDR=Clinical Dementia Rating scale; CDR-SOB=Clinical Dementia Rating – Sum of Boxes; PPVT=Peabody Picture Vocabulary Test; BNT=Boston Naming Test. Means (and standard deviations) included unless otherwise noted.
2.2. Neuropsychological Assessment
All participants completed an extensive neuropsychological battery, as described previously (Kramer et al., 2003). Global cognitive functioning was assessed with the Mini-Mental State Examination (MMSE) (Folstein et al., 1975). Given the predominance of language symptoms in svPPA, we quantified semantic knowledge using the Peabody Picture Vocabulary Task (PPVT) and confrontation naming using the 15-item Boston Naming Test (BNT), tests that are often impaired in svPPA (Mesulam et al., 2009). We have reported all clinical data that were analyzed as a part of the present study.
2.3. Laboratory Assessment of Emotion
2.3.1. Procedure
Participants’ emotional functioning was assessed at the UCSF Center for Psychophysiology and Behavior. Participants were seated in a comfortable chair in a well-lit experiment room. All stimuli were presented on a 21.5-inch computer monitor placed 4.25 feet from the participant. Participants were videotaped with a semi-obscured, remotely controlled video camera throughout the testing session. Participants were notified they would be recorded before the testing session began.
2.3.2. Emotional Reactivity Task
Participants completed a laboratory-based task of emotional reactivity. Before each trial, participants sat quietly during a 90-second resting baseline period during which an “X” was presented on a computer monitor. After each baseline period, they viewed a 90-second block of photographs (each photograph was shown for 15 seconds) that were designed to elicit a specific positive emotion (i.e., awe, nurturant love, or amusement), negative emotion (i.e., fear, sadness, or disgust), or no emotion (i.e., a neutral trial). All participants completed the seven trials in the same order. Images were obtained from the International Affective Picture System (Lang, 1997), previous studies (Shiota et al., 2011), and internet searches. Pilot testing in an independent sample of healthy adults indicated that these photographs elicited the target emotions.
After each trial, participants responded to a series of questions. First, they answered a multiple-choice question about the content of the images to ensure they had attended to and understood the stimuli. Next, participants rated their subjective experience of various positive emotions (amusement, awe, compassion, and love/tenderness) and negative emotions (fear, disgust, sadness, and surprise) during the trial on a Likert-type scale ranging from 0 (not at all) to 4 (extremely). To minimize working memory burden, the six photographs from the trial were presented again with the subjective experience questions. All questions were presented orally (through audio recordings) and visually (on the computer monitor). If a participant asked for clarification about the meaning of any words used in the testing session, the experimenter offered a brief, scripted definition as often as needed. Participants responded verbally to each question, and their answers were recorded by the experimenter.
Given that individuals with svPPA have semantic deficits and typically lose knowledge of less common words before they lose knowledge of more common words (Ogar et al., 2011), we used the English Lexicon Project Database (Balota et al., 2007) to ensure that the emotion words we used in the study were common in the English language (the emotion words ranged in frequency from log 7.5 – 12.5) and that the mean frequencies of the positive emotion (M=8.4, SD=1.5) and negative emotion (M=9.3, SD=2.15) words were similar. This step helped to ensure that, if people with svPPA exhibited atypical patterns in their verbally reported emotional experience, it would be unlikely that these results could be attributed to loss of semantic knowledge for less common emotion words.
2.4. Measures
2.4.1. Positive Emotional Experience
For each trial, we computed a positive emotional experience score by summing each participant’s subjective experience of the different positive emotions (i.e., amusement, awe, compassion, and tenderness/love). A total positive emotional experience score was then calculated for each participant by summing the positive emotional experience scores across all trials.
2.4.2. Smiling Behavior
We used the Facial Action Coding System (FACS), an objective approach to coding facial muscle movements (Ekman, 1997), to code so-called “Duchenne smiles” (i.e., smiles with cheeks raised and eyes engaged). A team of four certified FACS coders who were blind to the study hypotheses, diagnoses, and trial content rated the intensity of participants’ smiles on a second-by-second basis using a scale ranging from 1 (trace) to 5 (maximum). A code of 0 was given to seconds in which no smile was present. Twenty percent of the videos were coded by multiple coders to compute inter-rater consistency, and the coding system was found to be reliable (Krippendorf’s alpha=.81). We computed a smiling score for each trial by summing each participant’s second-by-second smiling intensity codes. A total smiling score was then calculated for each participant by summing the smiling scores across all of the trials. Two participants with bvFTD were not coded due to video recording issues and, thus, were not included in the smiling analyses. As the total smiling scores showed a strong positive skew, they were log transformed to improve approximation to the normal distribution.
2.5. Neuroimaging Acquisition and Preprocessing
2.5.1. Image Acquisition
Each participant underwent a 3T research quality structural MRI within 120 days of completing the emotional reactivity task. All MRIs were conducted at the UCSF Memory and Aging Center, using a 3T Magnetom VISION system (Siemens Inc., Iselin, N.J.) equipped with a standard quadrature head coil. A volumetric magnetization prepared rapid gradient echo MRI (MPRAGE) sequence (repetition time [TR]=10 milliseconds, echo-time [TE]=4 milliseconds, inversion time [TI]=300 milliseconds) was used to obtain T1-weighted images of the entire brain with a 15-degree flip angle, coronal orientation perpendicular to the double spin echo sequence, and 1.0 × 1.0 mm2 in-plane resolution and 1.5 mm slab thickness.
2.5.2. Image Preprocessing
The statistical parametric mapping (SPM12) software package running on MATLAB was used in the preprocessing steps for all images (https://www.fil.ion.ucl.ac.uk/spm/). SPM12 default parameters were utilized throughout with the exception of a light clean-up procedure, which was used in the morphological filtering step. Preprocessing involved segmenting the T1-structural images into gray matter, white matter, and cerebrospinal fluid and spatially normalizing them into MNI space. To optimize intersubject registration, each participant’s image was warped to a template created from 150 healthy older control participants. Spatially normalized, segmented, and modulated gray matter images were smoothed using an 8-mm FWHM isotropic Gaussian kernel. Each scan was visually examined for motion artifacts, and participants with motion were excluded from the analysis. Ten participants (seven patients with bvFTD and three patients with svPPA) were excluded due to motion artifacts. Thus, the neuroimaging analyses included a total of 30 patients (17 with bvFTD and 13 with svPPA).
2.5.3. Mask Preparation
To offset the loss of power that results from multiple comparison corrections, we masked our correlational neuroimaging analyses to the frontal and temporal lobes as well as subcortical and brainstem structures, regions that are vulnerable in FTD. The mask was created in SPM12 using the Wake Forest University PickAtlas toolbox with brain regions defined by the Automated Anatomical Labeling atlas.
2.6. Statistical Analyses
Statistical analyses were performed using R Studio (version 3.4.0). A Chi-square test of independence was used to examine whether there were group differences in the proportions of men and women. General linear models were used to compare age, education, disease severity (i.e., CDR total and CDR-SOB), general cognitive functioning (i.e., MMSE), semantic knowledge (e.g., PPVT), and confrontation naming (e.g., BNT) among the groups. Tukey-adjusted post hoc pairwise tests were used to follow up on significant results while correcting for multiple comparisons.
2.6.1. Validity Checks
For each trial, each participant’s response to the multiple-choice question regarding the content of the photographs was coded as correct or incorrect (if a participant did not provide a response, this was also coded as incorrect). Because many of the patients had language comprehension difficulties that could interfere with their ability to answer the content questions correctly, a laboratory staff member later reviewed the videos of the incorrect trials to ensure that errors on those trials were not due to participants paying inadequate attention to the stimuli.
2.6.2. Positive Emotional Experience and Smiling Behavior
We used linear mixed effects models to determine if there was a main effect of diagnosis or a diagnosis by trial interaction on total positive emotional experience or total smiling behavior (controlling for age and sex). A random intercept was specified for each participant. We planned to decompose any significant diagnosis by trial interactions with separate follow-up analyses of covariance (ANCOVAs) for each trial (same covariates). Tukey-adjusted post hoc tests were then used to identify significant pairwise differences between the groups. For trials in which total positive emotional experience differed among the groups, we also conducted follow-up ANCOVAS (controlling for age and sex) on the specific types of positive emotional experience (i.e., amusement, awe, love, and compassion) instead of the total score.
To confirm that any group differences in total positive emotional experience were not solely accounted for by verbal semantic deficits, we conducted a follow-up linear mixed effects model analysis in which we included PPVT as a covariate (in addition to age and sex). Given that psychiatric medications may impact emotional reactivity, we also conducted a follow-up analysis to ensure that psychiatric medication use did not influence our findings. We reviewed the participants’ medication records and coded (yes/no) whether they were taking a psychiatric medication for anxiety, depression, or psychosis. We then reran the mixed model analyses for total positive emotional experience and total smiling behavior but here included psychiatric medication use as an additional covariate (in addition to age and sex).
2.6.3. Neuroimaging Analyses
We conducted voxel-based morphometry (VBM) analyses using the voxel-based lesion-symptom mapping toolbox for MATLAB (Bates et al., 2003). To characterize the atrophy patterns of the clinical syndromes, we first compared the whole-brain gray matter maps of the svPPA and bvFTD groups to those of the healthy controls while controlling for age, sex, and total intracranial volume (TIV), to account for individual differences in head size.
We next ran general linear models in VBM to identify voxels in which smaller gray matter volume was associated with greater total positive emotional experience and greater total smiling behavior, masked to the regions described above (see 2.5.3. Mask Preparation). We conducted the correlational VBM analyses on the gray matter maps in the patients only because they had significant atrophy, and, thus, may have a disproportionate effect on the imaging results if analyzed with the healthy controls. We included age, sex, diagnosis (dummy coded 0 for svPPA and 1 for bvFTD), MMSE (to account for disease severity), and TIV as covariates in these analyses. We next ran additional VBM analyses in which we included PPVT or CDR-SOB scores as covariates (rather than MMSE) to examine whether any potential results held when we accounted for semantic deficits or behavioral symptoms, specifically. Finally, we investigated whether smaller gray matter volume was associated with lower total positive emotional experience or lower total smiling behavior in any regions.
Neuroimaging results are reported at p<.001, uncorrected, and at pFWE<.05. Corrections for multiple comparisons were addressed using permutation analyses. Statistical maps were calculated for 1000 random assignments of total positive emotional experience and total smiling behavior, and the fifth percentile of the maximum cluster size was applied as a threshold to the raw p<.001 map. We have used this combined peak and extent threshold permutation-based method in our prior imaging studies using similar methods in these clinical groups (Sturm et al., 2013; Wilson et al., 2010).
2.7. Data Availability
Data generated by the UCSF Memory and Aging Center are available upon request. All data requests can be submitted through the UCSF Memory and Aging Center Resource Request Form (http://memory.ucsf.edu/resources/data). Academic, not-for-profit investigators with Institutional Review Board approval from the UCSF Human Research Protection Program can request data for research studies. The UCSF Human Research Protection Program will not review the application until the UCSF Memory and Aging Center Executive Committee has signed off on the proposal and consent form. Data are not publicly available because they contain information that could compromise the privacy of the participants.
3. RESULTS
3.1. Demographics and Clinical Functioning
There were no differences among the groups in their education, F(2,56)=2.18, p=.12, or their proportions of men and women, χ2=(2, N=61)=2.91, p=.23. There was a main effect of group on age, F(2,58)=3.57, p=.03, however. Although follow-up Tukey tests indicated no pairwise differences, some comparisons (healthy controls vs. svPPA [p=.06] and healthy controls vs. bvFTD [p=.06]) approached significance. Although the bvFTD and svPPA groups were similarly impaired on the MMSE compared to the healthy controls, individuals with bvFTD were more impaired on the CDR Total and CDR-SOB than those with svPPA. As expected, individuals with svPPA had lower scores on semantic knowledge (i.e., PPVT) and confrontation naming (i.e., BNT) than those with bvFTD. There were similar proportions of patients in the bvFTD and svPPA groups taking psychiatric medications (Table 1).
3.2. Validity Checks
Across the entire sample, 90% of participants (76.2% of the svPPA group, 93.8% of bvFTD group, and 100% of the healthy controls) answered seven or more of the nine content questions correctly. There was no difference among the groups in the percentage of content questions they answered correctly, χ2(1, N=37)=2.01, p=.16.
3.3. Positive Emotional Experience and Smiling Behavior
There was a significant diagnosis by trial interaction on total positive emotional experience, F(12,56)=3.20, p<.001. Follow-up ANCOVAs indicated that individuals with svPPA reported significantly higher positive emotional experience than the healthy controls and people with bvFTD during the awe trial (svPPA>healthy controls: t(56)=4.22, p<.001; svPPA>bvFTD: t(56)=3.89, p<.001) and nurturant love trial (svPPA>healthy controls: t(56) =3.17, p=.01; svPPA>bvFTD: t(56)=3.25, p=.01), trials that typically elicit positive emotions (Figure 1). Individuals with svPPA also reported greater positive emotional experience during the neutral trial (svPPA>healthy controls: t(56)=3.28, p=.01; svPPA>bvFTD: t(56)=2.48, p=.04) and fear trial (svPPA>healthy controls, t(56)=2.61, p=.03) than the healthy controls and people with bvFTD, trials in which positive emotional experience is typically less often endorsed. There were no differences among the groups in positive emotional experience during the amusement, sadness, or disgust trials (Figure 1).
Figure 1. Mean positive emotional experience across trials in each diagnostic group.
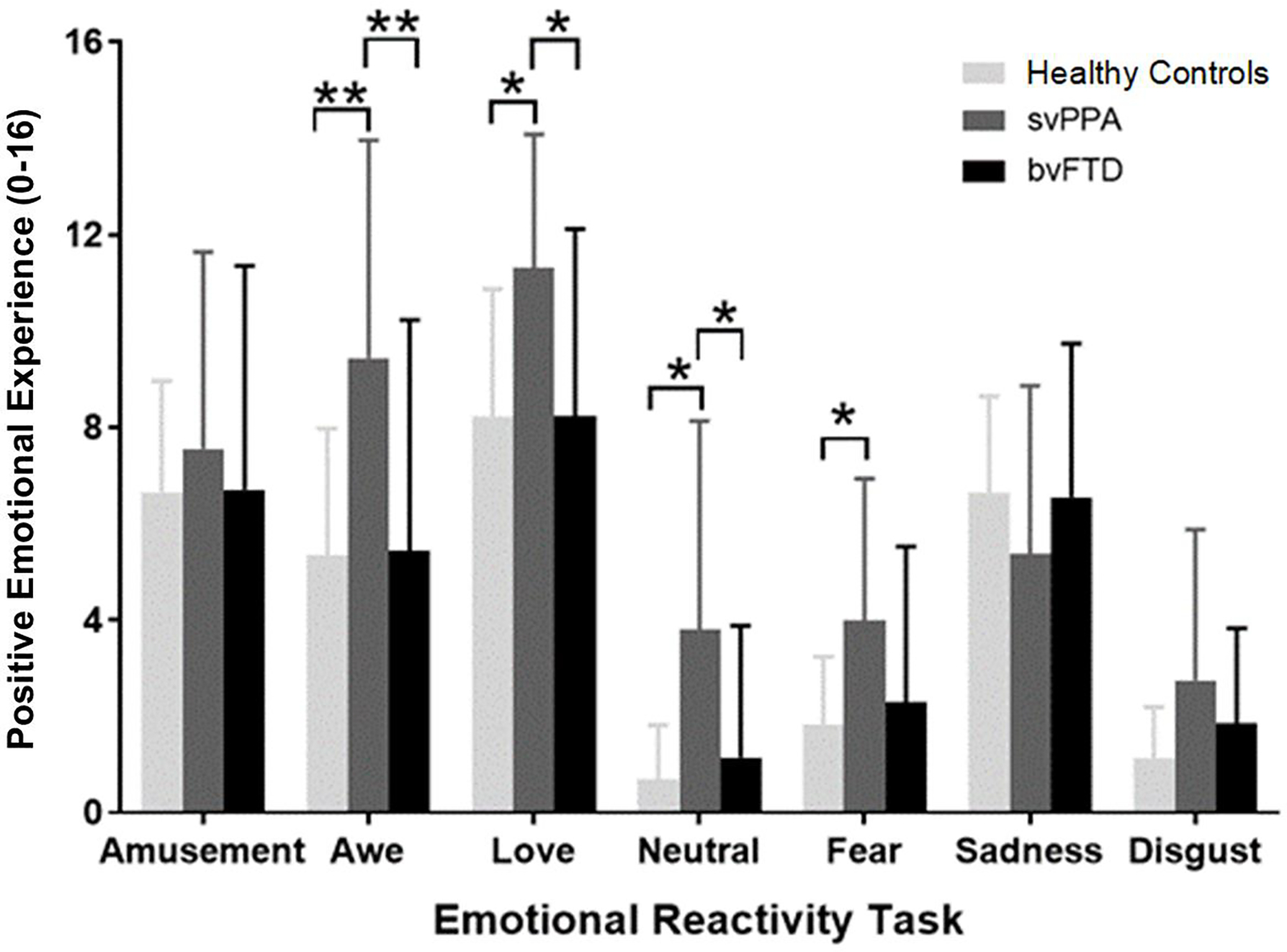
*= p<.05, **=p<.001. Separate ANCOVAs (controlling for age and sex) were run for each trial to decompose the significant diagnosis by trial interaction that was detected in our primary analysis on total positive emotional experience. Tukey-adjusted pairwise comparisons were conducted to correct for multiple comparisons. Error bars represent the standard error. These analyses revealed that people with svPPA reported significantly higher levels of total positive emotional experience during the awe (p<.001), love (p<.05), and neutral (p<.05) trials than the bvFTD and healthy control groups. People with svPPA also reported significantly higher levels of positive emotional experience during the fear trial than healthy controls (p<.05).
To determine whether people with svPPA reported higher levels of certain positive emotions more than others, we conducted additional ANCOVAs for those trials in which positive emotional experience was significantly elevated in svPPA. Compared to the healthy controls, individuals with svPPA reported greater amusement (p<.001), love/tenderness (p=.02), and compassion (p=.01) during the awe trial; greater awe (p=.01) during the love trial; greater awe (p=.02) and love (p<.01) during the neutral trial; and greater amusement (p=.02) and compassion (p=.01) during the fear trial (Table 2). Compared to individuals with bvFTD, those with svPPA reported greater amusement (p=.01) during the awe trial; greater awe (p=.01) and amusement (p=.01) during the love trial; greater awe (p=.01) and love (p=.01) during the neutral trial; and greater amusement (p=.02) during the fear trial (Table 2).
Table 2.
Positive emotional experience group comparisons.
| Healthy Controls M(SE) |
svPPA M(SE) |
bvFTD M(SE) |
Significance | |
|---|---|---|---|---|
|
| ||||
| Awe Trial | ||||
| Amusement Experience | 0.4 (1.0) | 2.2 (1.6) | 0.9 (1.3) | svPPA > Healthy controls** svPPA > bvFTD* |
| Awe Experience | 3.0 (0.8) | 2.7 (1.5) | 2.0 (1.6) | N.S. |
| Love Experience | 1.2 (1.1) | 2.4 (1.2) | 1.4 (1.7) | svPPA > Healthy controls* |
| Compassion Experience | 0.8 (1.0) | 2.1 (1.4) | 1.1 (1.5) | svPPA > Healthy controls* |
|
| ||||
| Love Trial | ||||
| Amusement Experience | 2.1 (1.1) | 3.1 (0.6) | 1.9 (1.5) | svPPA > bvFTD* |
| Awe Experience | 1.2 (1.0) | 2.3 (1.3) | 1.2 (1.4) | svPPA > Healthy controls* svPPA > bvFTD* |
| Love Experience | 2.9 (0.7) | 3.1 (0.9) | 2.6 (1.4) | N.S. |
| Compassion Experience | 2.0 (1.2) | 2.9 (1.0) | 2.6 (1.5) | N.S. |
|
| ||||
| Neutral Trial | ||||
| Amusement Experience | 0.3 (0.6) | 1.0 (1.3) | 0.5 (1.0) | N.S. |
| Awe Experience | 0.1 (0.3) | 0.8 (1.2) | 0.1 (0.4) | svPPA > Healthy controls* svPPA > bvFTD* |
| Love Experience | 0.2 (0.5) | 1.2 (1.3) | 0.2 (0.7) | svPPA > Healthy controls* svPPA > bvFTD* |
| Compassion Experience | 0.1 (0.5) | 0.9 (1.3) | 0.4 (1.2) | N.S. |
|
| ||||
| Fear Trial | ||||
| Amusement Experience | 0.5 (0.7) | 1.4 (1.3) | 0.5 (1.0) | svPPA > Healthy controls * svPPA > bvFTD* |
| Awe Experience | 1.1 (0.7) | 1.1 (1.0) | 0.8 (1.1) | N.S. |
| Love Experience | 0.0 (0.0) | 0.8 (1.2) | 0.6 (1.4) | N.S. |
| Compassion Experience | 0.2 (0.5) | 1.0 (0.8) | 0.4 (1.0) | svPPA > Healthy controls * |
=p<.05
=p<.001, mean (standard error).
Follow-up ANCOVAs (controlling for age and sex) for each type of positive emotional experience. N.S. indicates non-significant.
There was also a significant diagnosis by trial interaction on smiling behavior, F(12,54)=2.06, p=.02. Follow-up ANCOVAs revealed that individuals with svPPA exhibited greater smiling behavior than those with bvFTD during the fear, t(54)=2.86, p=.02, and sadness, t(54)=2.52, p=.04, trials. Individuals with bvFTD, in contrast, displayed lower smiling behavior than the healthy controls during the amusement trial, t(54)=−2.42, p<.05; Figure 2).
Figure 2. Mean smiling behavior across trials in each diagnostic group.
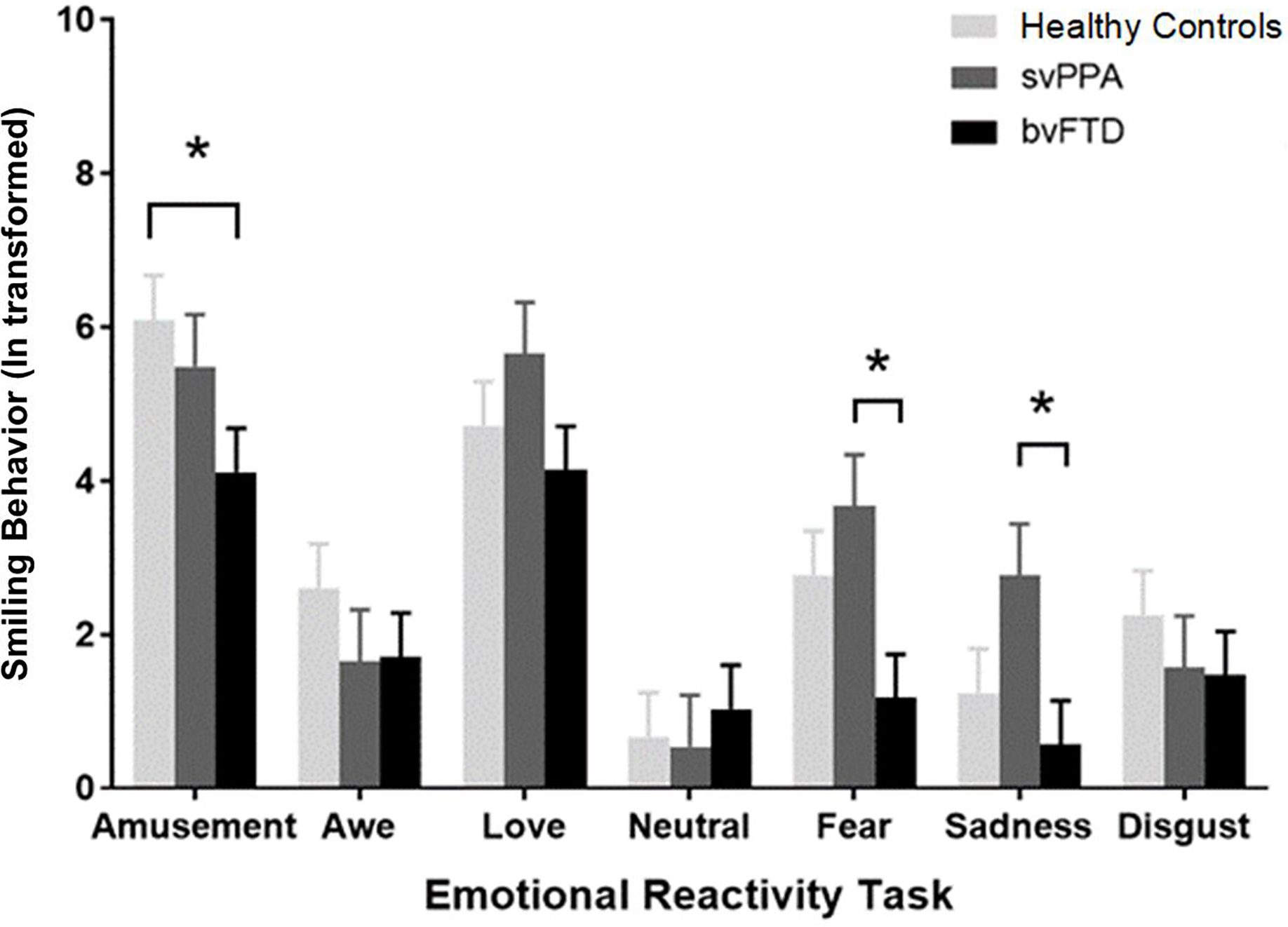
*=p<.05. Separate ANCOVAs (controlling for age and sex) were run for each trial to decompose the significant diagnosis by trial interaction that was detected in our primary analysis on smiling behavior. Tukey-adjusted pairwise comparisons were used to correct for multiple comparisons. People with svPPA had a higher total smiling score during the fear (p<.05) and sadness (p<.05) trials than those with bvFTD. People with bvFTD had a greater total smiling score during the amusement trial than the healthy controls (p<.05).
3.4. Follow-Up Analyses
We conducted follow-up analyses to ensure that the enhanced positive emotional experience that we detected in svPPA was not accounted for by other factors. First, we reran the mixed model analysis on total positive emotional experience but here included PPVT (in addition to age and sex) as a covariate. The diagnosis by trial interaction remained significant, F(12,50)=3.31, p<.001), suggesting that individuals with svPPA reported higher levels of positive emotional experience even when accounting for verbal semantic deficits.
We then reran the mixed model analyses on total positive emotional experience and total smiling behavior but here included psychiatric medication use (in addition to age and sex) as a covariate. The diagnosis by trial interactions on total positive emotional experience, F(12,55)=3.20, p<.001, and total smiling behavior, F(12,53)=2.06, p=.02, remained significant, which suggested that psychiatric medication use did not account for our main results.
3.5. Neuroimaging Analyses
3.5.1. Group Comparisons
The group comparisons revealed that, as expected, there was left-lateralized atrophy in the anterior temporal lobe, inferior temporal gyrus, insula, anterior cingulate cortex, amygdala, hippocampus, and caudate in svPPA when compared to the healthy controls (pFWE<.05). In bvFTD, there was predominant atrophy in bilateral anterior insula, orbitofrontal cortex, anterior cingulate cortex, anterior temporal lobe, amygdala, and caudate compared to the healthy controls (pFWE<.05). Both clinical groups had overlapping atrophy in a priori regions of interest including the left temporal lobe and insula, among other regions (Figure 3).
Figure 3. Distinct and overlapping atrophy patterns in svPPA and bvFTD.
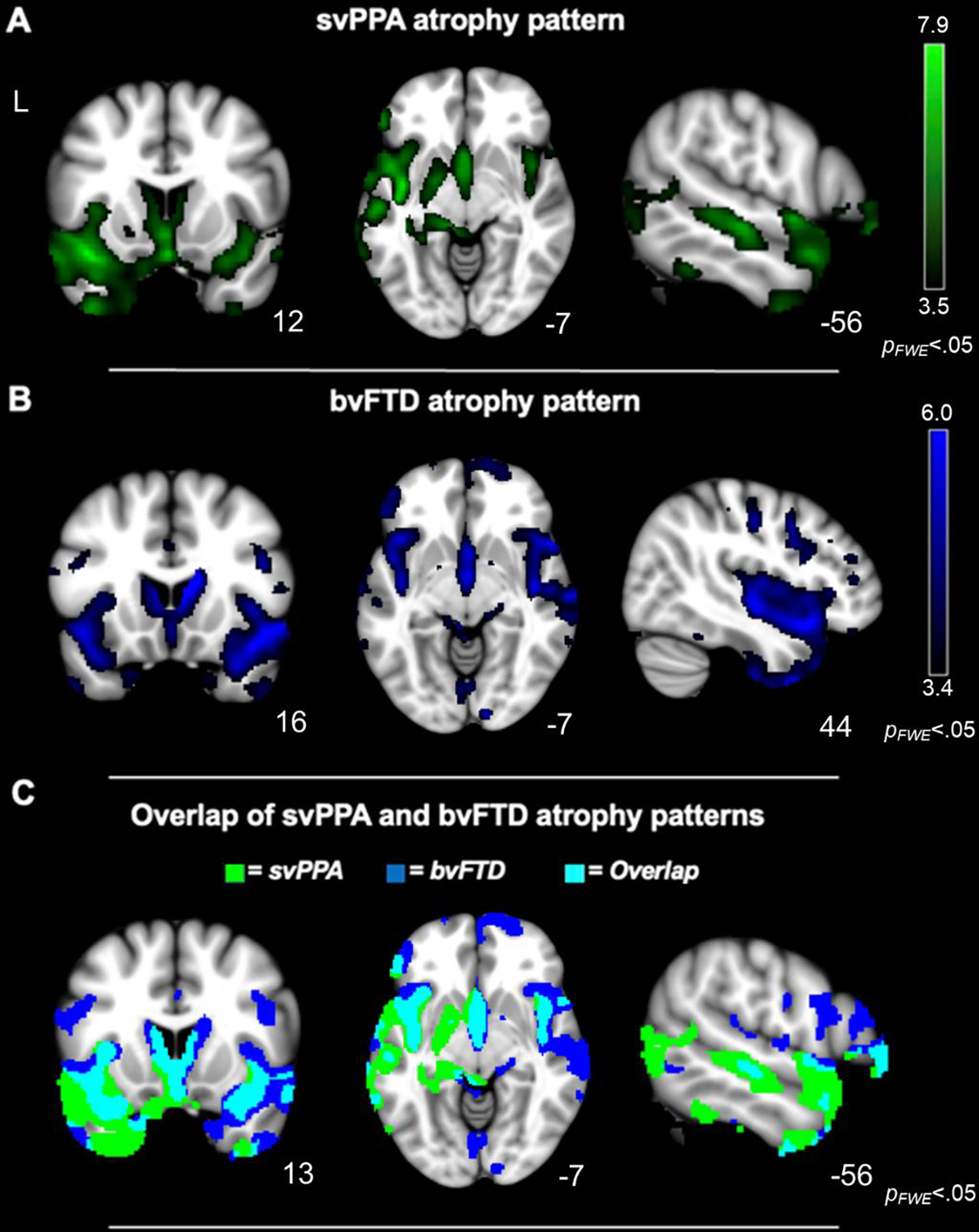
Whole-brain voxel-based morphometry analyses (controlling for age, sex, and TIV) confirmed that the A) svPPA and (B) bvFTD groups had smaller gray matter volume than the healthy controls in frontotemporal and subcortical regions typically atrophied in these syndromes (pFWE<.05). (C) In svPPA and bvFTD, there was some overlapping atrophy, but the people with svPPA tended to have more left lateralized atrophy than those with bvFTD, whose atrophy was more bilateral (pFWE<.05). Statistical maps are superimposed on the Montreal Neurological Institute template.
3.5.1. Neural Correlates of Positive Emotional Reactivity
At the strictest statistical threshold (pFWE<.05), smaller gray matter volume in two clusters in the left superior temporal gyrus (STG) was associated with greater total positive emotional experience and greater total smiling behavior in the patients (Table 3 and Figure 4). Although there was overlap between these clusters, greater positive emotional experience was more strongly associated with smaller gray matter volume in left middle STG, but greater smiling was more strongly associated with smaller gray matter volume in left posterior STG. At p<.001, smaller volume in bilateral middle frontal gyri, left fusiform, and left inferior temporal gyrus was also associated with greater total positive emotional experience and greater total smiling behavior. Although some shared regions were associated with both experience and behavior, each emotional reactivity measure also had other non-overlapping neural correlates. While greater positive emotional experience was associated with smaller gray matter volume in the left dorsal anterior insula, left ventrolateral prefrontal cortex, left inferior temporal gyrus, and left fusiform, among other regions (p<.001), greater smiling behavior was uniquely associated with smaller volume in bilateral precentral gyri, bilateral supplementary motor area, bilateral superior frontal gyri, among others (p<.001). No regions emerged in which smaller gray matter volume was associated with lower positive emotional experience or lower smiling behavior at this threshold.
Table 3.
Neural correlates of total positive emotional experience and total smiling behavior.
| Anatomical region | Cluster volume (mm3) | MNI coordinates | Maximum T-score | p-value | ||
|---|---|---|---|---|---|---|
|
|
||||||
| x | y | z | ||||
|
| ||||||
| Total Positive Emotional Experience | ||||||
| Left Superior Temporal Gyrus (mid) | 1559 | −56 | −20 | −3 | 5.88 | .04* |
| †Left Middle Temporal Gyrus | ||||||
| Left Dorsal Anterior Insula | 368 | −30 | 29 | 11 | 4.91 | .17 |
| †Left Pars Triangularis | ||||||
| Midbrain | 304 | −6 | −18 | −9 | 4.10 | .20 |
| Right Middle Frontal Gyrus | 253 | 35 | 42 | 12 | 5.13 | .23 |
| Left Middle Temporal Gyrus | 223 | −51 | −56 | 6- | 4.48 | .26 |
| Left Middle Frontal Gyrus | 192 | −33 | 39 | 17 | 4.48 | .29 |
| Left Middle Frontal Gyrus | 182 | −23 | 33 | 29 | 4.79 | .30 |
| Left Fusiform Gyrus | 115 | −33 | −26 | −20 | 4.13 | .41 |
| Left Inferior Temporal Gyrus | 105 | −57 | −15 | −35 | 3.93 | .44 |
| Left Orbitofrontal Cortex | 91 | −32 | 48 | −8 | 4.36 | .47 |
| Left Amygdala | 91 | −29 | −2 | −17 | 4.12 | .47 |
| Left Inferior Temporal Gyrus | 78 | −59 | −44 | −18 | 3.82 | .51 |
| Left Inferior Temporal Gyrus | 64 | −42 | −54 | −8 | 4.18 | .54 |
| Left Middle Frontal Gyrus | 57 | −36 | 21 | 38 | 4.09 | .56 |
| Left Middle Temporal Gyrus | 57 | −60 | −48 | 0 | 3.97 | .56 |
| Left Olfactory Bulb | 54 | −24 | 8 | −14 | 3.74 | .57 |
| Left Middle Temporal Gyrus | 54 | −54 | −6 | −24 | 3.76 | .57 |
| Left Parahippocampal Gyrus | 54 | −21 | −12 | −29 | 3.68 | .57 |
| Right Midbrain | 47 | 14 | −21 | −9 | 4.56 | .60 |
| Left Inferior Temporal Gyrus | 47 | −56 | −2 | −35 | 3.59 | .59 |
| Right Middle Frontal Gyrus | 44 | 20 | 42 | 24 | 4.77 | .60 |
| Left Pars Opercularis | 37 | −38 | 11 | 14 | 3.83 | .62 |
| Left Midbrain | 30 | −12 | −23 | −9 | 4.01 | .64 |
| Left Putamen | 27 | −35 | −17 | −8 | 4.18 | .66 |
| Right Superior Temporal Gyrus | 17 | 44 | −24 | 2 | 3.77 | .70 |
| Left Inferior Temporal Gyrus | 17 | −41 | −45 | −9 | 3.83 | .696 |
| Right Pons | 17 | 11 | −33 | −30 | 3.81 | .696 |
| Total Smiling Behavior | ||||||
| Left Superior Temporal Gyrus (posterior) | 1232 | −53 | −41 | 11 | 5.65 | .04* |
| Left Inferior Temporal Gyrus | 618 | −48 | −35 | −24 | 5.3 | .10 |
| Left Middle Frontal Gyrus | 452 | −36 | 23 | 42 | 4.67 | .13 |
| Right Middle Frontal Gyrus | 233 | 39 | 41 | 20 | 4.25 | .24 |
| Right Posterior Midcingulate Cortex | 145 | 3 | −35 | 50 | 4.44 | .36 |
| Left Middle Frontal Gyrus | 108 | −30 | 8 | 57 | 4.51 | .42 |
| Brainstem | 105 | 15 | −35 | −33 | 4.52 | .43 |
| Right Superior Frontal Gyrus | 74 | 20 | 12 | 51 | 4.50 | .52 |
| Left Superior Frontal Gyrus | 64 | −15 | 48 | 5 | 4.35 | .52 |
| Right Precentral Gyrus | 57 | 45 | 2 | 44 | 4.14 | .56 |
| Right Medial Orbitofrontal Cortex | 43.9 | 12 | 57 | −9 | 3.82 | .626 |
| Left Fusiform Gyrus | 37.1 | −41 | −47 | −11 | 3.87 | .651 |
| Right Middle Frontal Gyrus | 30.4 | 33 | 21 | 36 | 3.73 | .680 |
| Left Precentral Gyrus | 30.4 | −51 | 14 | 33 | 3.68 | .680 |
| Right Middle Frontal Gyrus | 27.0 | 54 | 18 | 39 | 3.72 | .691 |
| Left Middle Frontal Gyrus | 27.0 | −29 | 32 | 29 | 3.98 | .691 |
| Right Supplementary Motor Area | 20.3 | 18 | 15 | 66 | 3.85 | .719 |
| Left Inferior Temporal Gyrus | 20.3 | −59 | −44 | −18 | 3.7 | .719 |
| Right Superior Frontal Gyrus | 16.9 | 21 | −2 | 74 | 3.67 | .734 |
| Left Supplementary Motor Area | 16.9 | −11 | −5 | 69 | 3.85 | .734 |
| Left Precentral Gyrus | 16.9 | −47 | 0 | 44 | 3.64 | .734 |
| Left Ventral Anterior Insula | 16.9 | −26 | 11 | −15 | 3.61 | .734 |
Voxel-based morphometry analyses (controlling for age, sex, diagnosis, MMSE, and TIV) revealed that smaller gray matter volume in predominately left hemisphere regions was associated with greater total positive emotional experience and greater total smiling behavior. Montreal Neurological Institute coordinates (x, y, z) are reported for maximum T-score in each cluster. All results are significant at p<.001, uncorrected.
=Results significant at pFWE<.05.
=region included in cluster above. Clusters smaller than 15 mm3 were excluded.
Figure 4. Left-lateralized atrophy related to greater total positive emotional experience and greater total smiling behavior.
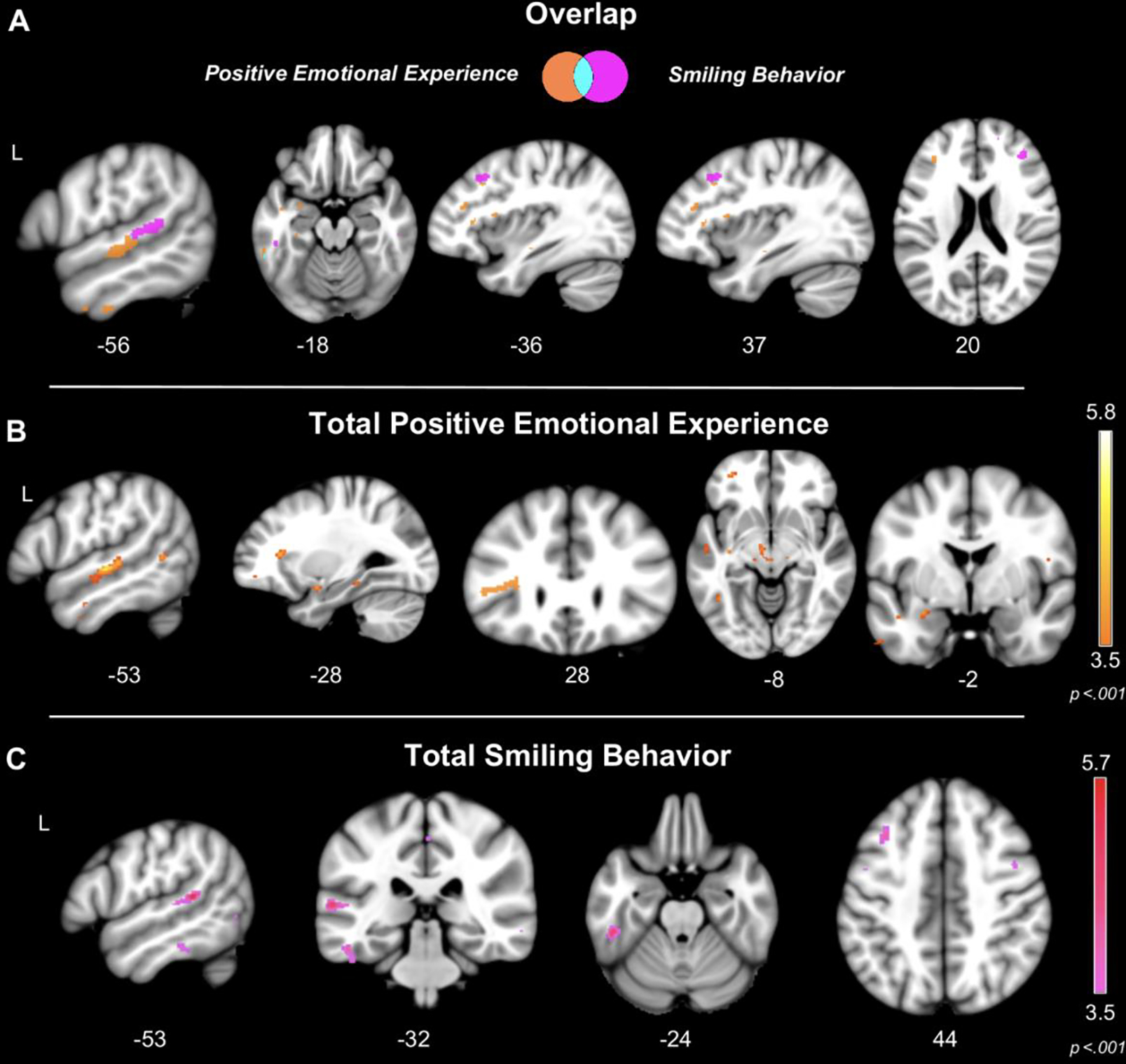
Voxel-based morphometry analyses (controlling for age, sex, diagnosis, MMSE, and TIV) revealed that (A) smaller gray matter volume in left superior temporal gyrus was associated with greater total positive emotional experience (pFWE<.05) and greater total smiling behavior (pFWE<.05). (B) Greater total positive emotional experience was associated with smaller gray matter volume in the left superior temporal gyrus, left dorsal anterior insula, and left orbitofrontal cortex (p<.001), and (C) greater total smiling behavior was associated with smaller gray matter volume in the left temporal lobe and middle frontal gyrus. Color bars represent T-scores at p<.001. Statistical maps are superimposed on the Montreal Neurological Institute template.
3.5.2. Follow-Up Neuroimaging Analyses
In follow-up VBM analyses that included PPVT or CDR-SOB scores as covariates to account for disease severity (instead of MMSE), our main neuroimaging results persisted, albeit with smaller cluster sizes. At the strictest statistical threshold (pFWE<.05), greater total positive emotional experience continued to correlate with smaller gray matter volume in the left STG when controlling for either PPVT or CDR-SOB. At the uncorrected threshold of p<.001, both greater total positive emotional experience and greater total smiling behavior were associated with smaller volume in left STG when controlling for either of these covariates (Supplementary Figure 1).
To ensure the VBM results reflected linear relationships between gray matter volume and positive emotional reactivity across the sample (and were not driven by diagnostic group), we extracted the gray matter volume from the left STG and the left dorsal anterior insula clusters that correlated with total positive emotional experience. We entered these cluster volumes (as well as age, sex, diagnosis, TIV, and MMSE) in a regression model and found that volume in both the left STG, t(22)=−3.92, p<.001), and the left dorsal anterior insula, t(22)=−2.94, p<.01, were significant predictors of total positive emotional experience. Scatterplots further confirmed that there were linear relationships between gray matter volume in these regions and positive emotional experience across both clinical groups (Figure 5).
Figure 5. Linear association between total positive emotional experience and gray matter volume across the clinical groups.
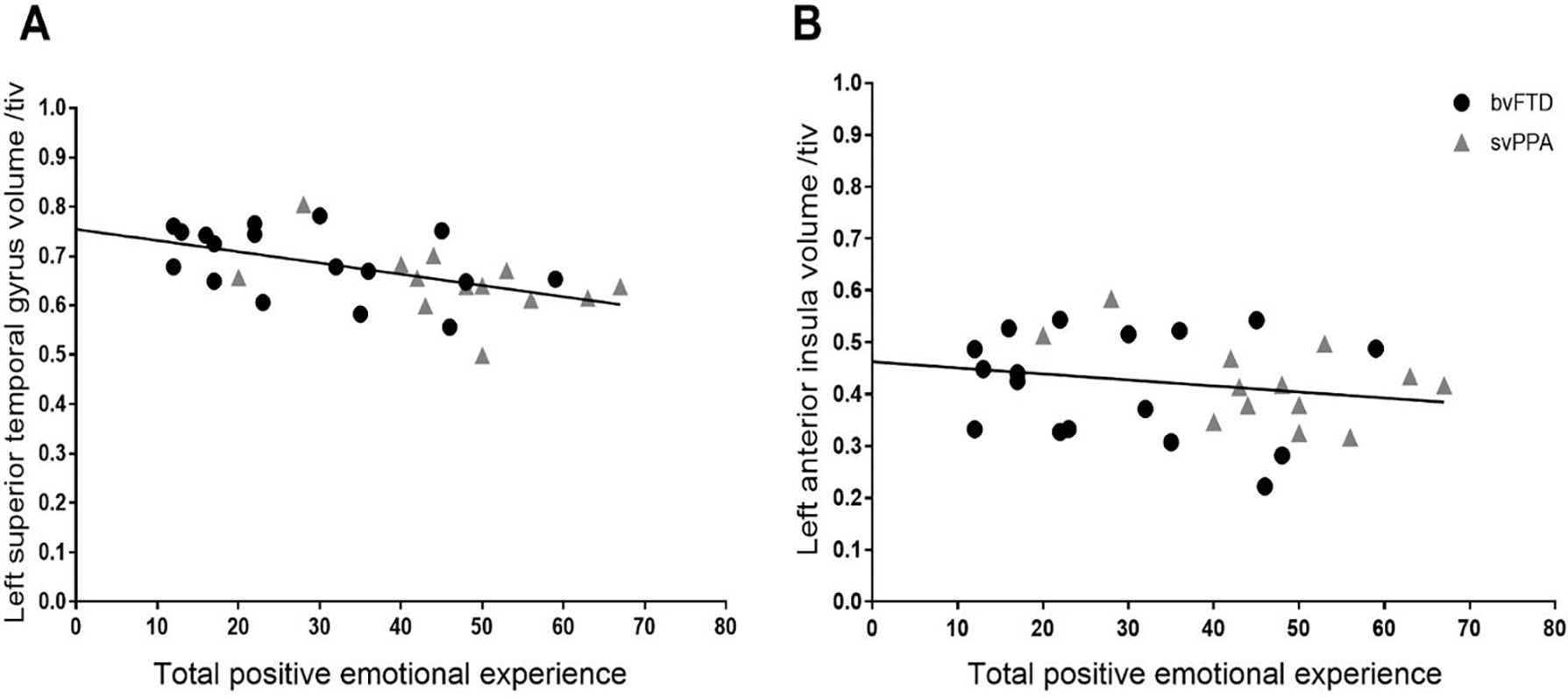
A regression model (controlling for age, sex, diagnosis, TIV, and MMSE) confirmed that total positive emotional experience had a linear association across the clinical groups with gray matter volume in the left superior temporal gyrus and left dorsal anterior insula clusters that emerged in the VBM analyses. These results suggest the neuroimaging results reflected linear brain-emotion associations and were not driven by one diagnostic group.
4. Discussion
Our results indicate positive emotional reactivity is elevated in svPPA. Individuals with svPPA reported greater positive emotional experience and exhibited greater smiling behavior than the bvFTD and healthy control groups in response to a variety of affective, and non-affective, stimuli. Compared to the other groups, people with svPPA reported higher levels of positive emotional experience while viewing photographs that typically elicit positive emotions (e.g., awe and nurturant love trials). They also, however, reported greater positive emotional experience to photographs that usually elicit negative emotions (e.g., fear trial) and even to photographs that generally elicit no emotions at all (e.g., neutral trial). The elevated positive emotional experience in svPPA was not accounted for by semantic deficits alone (i.e., our results held when controlling for PPVT) and did not appear to reflect variation in word frequency (i.e., people with svPPA did not systematically endorse emotion words that are more or less common in the English language). Behaviorally, whereas individuals with bvFTD smiled less than the healthy controls during the amusement trial, those with svPPA exhibited greater smiling than those with bvFTD during the fear and sadness trials. While smaller gray matter volume in the left STG, left inferior temporal gyrus, left fusiform gyrus, and bilateral middle frontal gyri was associated with both greater positive emotional experience and greater smiling behavior, the experiential and behavioral measures also had unique neural correlates that included overlapping yet distinct neural systems predominantly in the left hemisphere.
Relatively little is known about social and emotional alterations in svPPA, but the present study suggests positive emotions are elevated in this FTD subtype. In bvFTD, right-sided atrophy is often associated with affective blunting and diminished emotional reactivity (Bickart et al., 2014; Sturm et al., 2013; Verstaen et al., 2016), though some patients do exhibit elevated positivity (Clark et al., 2015; Hua et al., 2018; Perry et al., 2017). By measuring experiential and behavioral responses to a wide range of stimuli, we were able to uncover elevated positive emotional reactivity in svPPA in a variety of contexts, which is consistent with one prior study of facial expressivity in this disorder (Kumfor et al., 2019). The enhanced positive reactions in svPPA not only arose in response to stimuli intended to elicit positive emotions (e.g., smiling babies and nature scenes) but also in response to those that typically evoke negative emotions (e.g., snakes and spiders) or even no emotions at all (e.g., power cords and a sink).
Our results build on previous work and suggest positive emotions may become dysregulated in FTD when atrophy targets frontotemporal systems in the left hemisphere. In a previous study of FTD, we found atrophy in left-lateralized in regions that support emotion regulation, including dorsal anterior insula and ventrolateral prefrontal cortex, was associated with greater positive emotional reactivity (Sturm et al., 2015). The present study offers convergent evidence that smaller gray matter volume in these regions underlies patients’ heightened positive emotional reactivity and suggests the left temporal lobe also plays a critical role in modulating positive emotional experience and behavior. The left STG, fusiform gyrus, inferior temporal gyrus, and middle frontal gyri emerged as regions in which atrophy was associated with greater positive emotional experience and smiling behavior. The STG plays a central role in auditory comprehension and language (Price, 2010) and is critical for processing socioemotional cues including facial expressions, eye gaze, mouth movements, gestures, and prosody (Adolphs, 2003; Gobbini et al., 2004; Haxby et al., 2000; Mion et al., 2010). By integrating multiple streams of sensory information (Beauchamp et al., 2004; Calvert et al., 2000), the STG may help to fine tune activity in emotion generation systems (Saper, 2002) and to produce context-appropriate emotional reactions (Müller et al., 2012; Picó-Pérez et al., 2018). Left-lateralized STG degeneration, in particular, may disrupt appraisal processes and interfere with patients’ comprehension of socioemotional signals and may increase positive emotional reactivity by releasing activity in emotion generators (Lieberman et al., 2011; Marshall et al., 2018; McRae et al., 2012) such as the left amygdala (Müller et al., 2012). Although atrophy in the left amygdala was also associated with greater positive emotional experience, elevated positive emotional reactivity in the patients may also require relative preservation of other emotion generators in the left hemisphere or participation of homologous structures in the right. In svPPA, degeneration of the left STG may also disrupt vulnerable left-lateralized white matter tracts (Agosta et al., 2013) that connect the STG to ventrolateral prefrontal cortex (Kiernan, 2012; Öngür et al., 2003; Petrides & Pandya, 1988, 2007, 2009a, 2009b), a region critical for emotion regulation (Lieberman et al., 2007) and behavioral inhibition (Aron et al., 2004). Atrophy of bilateral middle frontal gyri, which also support cognitive control and emotion regulation (Etkin et al., 2015; Kohn et al., 2014; Ochsner & Gross, 2005; Silvers et al., 2012), likely also contributed to enhanced positive emotional reactivity in FTD by reducing the modulation of positivity across contexts.
As multisystem responses, emotions are accompanied by alterations in experience and behavior, among other changes (Levenson, 2003). Although the experiential and behavioral components of positive emotions are typically linked (Mauss et al., 2005), it is possible that heightened positive emotional experience and smiling behavior in svPPA may result from atrophy in distinct, yet overlapping, left-lateralized neural systems. Whereas greater positive emotional experience was associated with atrophy in the left middle STG and frontal regions that support behavioral control and emotion regulation (e.g., left dorsal anterior insula and left ventrolateral prefrontal cortex), elevated smiling behavior was associated with atrophy in left posterior STG and more posterior frontal regions that support motor functioning (e.g., bilateral precentral gyri and bilateral supplementary motor area). It is possible that degeneration of the left middle STG, which has strong connections with both dorsal anterior insula (Cauda et al., 2011) and ventrolateral prefrontal cortex (Erickson et al., 2017; Margulies & Petrides, 2013), may disrupt behavioral inhibition and cognitive control, thereby disinhibiting positive thoughts and feelings and enhancing positive emotional experience. The posterior STG, although it also has strong connections with ventrolateral prefrontal cortex, is tightly connected with the precentral gyrus, supplementary motor area, and middle frontal gyrus (Erickson et al., 2017). Thus, left-lateralized posterior STG atrophy may have more dramatic effects on facial movements and release smiling behavior, in particular, by disinhibiting bilateral frontal regions that support facial motor control (Margulies & Petrides, 2013; Petrides et al., 2005).
The present study has several limitations to consider. First, we used static images to evoke emotional reactivity. Future studies that examine reactivity to dynamic positive emotional stimuli, which may be more powerful elicitors of emotion, would help to further elucidate the nature of the positive emotional alterations in FTD. Second, emotional experience is subjective and, thus, can only be assessed with language-based measures. We found elevated positive emotional experience in individuals with svPPA who, by definition, have impaired semantic knowledge. We attempted to rule out the possibility that the heightened positive emotional experience we found in svPPA was not due to semantic deficits alone (e.g., by examining the frequency of the emotion terms and whether positive emotional experience related to semantic knowledge), but it is possible that loss of semantic knowledge may somehow have caused individuals with svPPA to inflate their endorsement of positive emotion terms more than negative ones. Third, we used MMSE as a measure of disease severity in our main neuroimaging analyses, but this measure is not ideal for capturing disease severity in both clinical groups. When we included covariates to account for semantic deficits (PPVT) and behavioral symptoms (CDR-SOB)—clinical measures that sensitive to disease progression in svPPA and bvFTD, respectively—the associations that left STG had with total positive emotional experience and total smiling behavior remained significant, though the clusters were much smaller. It is likely that alterations in positive emotions are indeed related to disease severity in FTD and to other language and behavioral symptoms. Future studies that investigate whether positive emotions change with disease progression will be needed to advance our understanding of the natural history of positive emotional reactivity in FTD.
FTD syndromes offer a unique opportunity to study the neural systems that produce and control positive emotions (Levenson, 2019; Levenson et al., 2014). Although some theorists posit the right hemisphere is essential for all emotions (Gainotti, 1972, 2019), our results are more aligned with neuroanatomical models in which the left hemisphere plays a predominant role in the generation and regulation of positive emotions (Davidson, 1992). Consistent with our previous work, we found elevated positive emotional reactivity in svPPA, which related to leftward frontotemporal atrophy patterns. This study helps to expand our understanding of the nature of positive emotion alterations in FTD and their underlying neural correlates.
Supplementary Material
Acknowledgements
We are grateful to our participants and their families for participating in this research.
Funding
This research was supported by the National Institute on Aging K23AG040127, R01AG057204, R01AG052496 (PI: Sturm); P01 AG1972403 (PI: Miller), P50 AG02350 (PI: Miller), and the Larry L. Hillblom Foundation.
Abbreviations:
- bvFTD
behavioral variant frontotemporal dementia
- CDR-SOB
Clinical Dementia Rating – Sum of Boxes
- FTD
frontotemporal dementia
- MMSE
Mini-Mental State Examination
- PPVT
Peabody Picture Vocabulary Test
- SPM
statistical parametric mapping
- svPPA
semantic variant primary progressive aphasia
- STG
superior temporal gyrus
- TIV
total intracranial volume
- VBM
voxel-based morphometry
Footnotes
Competing Interests
The authors report no competing interests.
References
- Adolphs R (2003). Cognitive neuroscience: Cognitive neuroscience of human social behaviour. Nature Reviews Neuroscience, 4(3), 165–178. 10.1038/nrn1056 [DOI] [PubMed] [Google Scholar]
- Agosta F, Galantucci S, Canu E, Cappa SF, Magnani G, Franceschi M, Falini A, Comi G, & Filippi M (2013). Disruption of structural connectivity along the dorsal and ventral language pathways in patients with nonfluent and semantic variant primary progressive aphasia: A DT MRI study and a literature review. Brain and Language, 127(2), 157–166. 10.1016/j.bandl.2013.06.003 [DOI] [PubMed] [Google Scholar]
- Aron AR, Robbins TW, & Poldrack RA (2004). Inhibition and the right inferior frontal cortex. In Trends in Cognitive Sciences (Vol. 8, Issue 4, pp. 170–177). 10.1016/j.tics.2004.02.010 [DOI] [PubMed] [Google Scholar]
- Balota DA, Yap MJ, Cortese MJ, Hutchison KA, Kessler B, Loftis B, Neely JH, Nelson DL, Simpson GB, & Treiman R (2007). The english lexicon project. In Behavior Research Methods (Vol. 39, Issue 3, pp. 445–459). Springer; New York LLC. 10.3758/BF03193014 [DOI] [PubMed] [Google Scholar]
- Beauchamp MS, Lee KE, Argall BD, & Martin A (2004). Integration of auditory and visual information about objects in superior temporal sulcus. Neuron, 41(5), 809–823. 10.1016/S0896-6273(04)00070-4 [DOI] [PubMed] [Google Scholar]
- Bickart KC, Brickhouse M, Negreira A, Sapolsky D, Barrett LF, & Dickerson BC (2014). Atrophy in distinct corticolimbic networks in frontotemporal dementia relates to social impairments measured using the social impairment rating scale. Journal of Neurology, Neurosurgery and Psychiatry, 85(4), 438–448. 10.1136/jnnp-2012-304656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binney RJ, Henry ML, Babiak M, Pressman PS, Santos-Santos MA, Narvid J, Mandelli ML, Strain PJ, Miller BL, Rankin KP, Rosen HJ, & Gorno-Tempini ML (2016). Reading words and other people: A comparison of exception word, familiar face and affect processing in the left and right temporal variants of primary progressive aphasia. Cortex, 82, 147–163. 10.1016/j.cortex.2016.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borghesani V, Narvid J, Battistella G, Shwe W, Watson C, Binney RJ, Sturm VE, Miller Z, Mandelli ML, Miller B, & Gorno-Tempini ML (2019). “Looks familiar, but I do not know who she is”: The role of the anterior right temporal lobe in famous face recognition. Cortex, 115, 72–85. 10.1016/j.cortex.2019.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J, Deng J, Neuhaus J, Sible IJ, Sias AC, Lee SE, Kornak J, Marx GA, Karydas AM, Spina S, Grinberg LT, Coppola G, Geschwind DH, Kramer JH, Gorno-Tempini ML, Miller BL, Rosen HJ, & Seeley WW (2019). Patient-Tailored, Connectivity-Based Forecasts of Spreading Brain Atrophy. SSRN Electronic Journal. 10.2139/ssrn.3339908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvert GA, Campbell R, & Brammer MJ (2000). Evidence from functional magnetic resonance imaging of crossmodal binding in the human heteromodal cortex. Current Biology, 10(11), 649–657. 10.1016/S0960-9822(00)00513-3 [DOI] [PubMed] [Google Scholar]
- Cauda F, D’Agata F, Sacco K, Duca S, Geminiani G, & Vercelli A (2011). Functional connectivity of the insula in the resting brain. NeuroImage, 55(1), 8–23. 10.1016/j.neuroimage.2010.11.049 [DOI] [PubMed] [Google Scholar]
- Cavanaugh LA, Bettman JR, & Luce MF (2015). Feeling love and doing more for distant others: Specific positive emotions differentially affect prosocial consumption. Journal of Marketing Research, 52(5), 657–673. 10.1509/jmr.10.0219 [DOI] [Google Scholar]
- Clark CN, Nicholas JM, Gordon E, Golden HL, Cohen MH, Woodward FJ, MacPherson K, Slattery CF, Mummery CJ, Schott JM, Rohrer JD, & Warren JD (2015). Altered Sense of Humor in Dementia. Journal of Alzheimer’s Disease, 49(1), 111–119. 10.3233/JAD-150413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins JA, Montal V, Hochberg D, Quimby M, Mandelli ML, Makris N, Seeley WW, Gorno-Tempini ML, & Dickerson BC (2017). Focal temporal pole atrophy and network degeneration in semantic variant primary progressive aphasia. Brain, 140(2), 457–471. 10.1093/brain/aww313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson RJ (1992). Emotion and Affective Style: Hemispheric Substrates. Psychological Science, 3(1), 39–43. 10.1111/j.1467-9280.1992.tb00254.x [DOI] [Google Scholar]
- Eckart JA, Sturm VE, Miller BL, & Levenson RW (2012). Diminished disgust reactivity in behavioral variant frontotemporal dementia. Neuropsychologia, 50(5), 786–790. 10.1016/j.neuropsychologia.2012.01.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson LC, Rauschecker JP, & Turkeltaub PE (2017). Meta-analytic connectivity modeling of the human superior temporal sulcus. Brain Structure and Function, 222(1), 267–285. 10.1007/s00429-016-1215-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A, Büchel C, & Gross JJ (2015). Neural Correlates of Emotion Regulation. Nature Publishing Group, 16. 10.1038/nrn4044 [DOI] [Google Scholar]
- Fittipaldi S, Ibanez A, Baez S, Manes F, Sedeno L, & Garcia AM (2019). More than words: Social cognition across variants of primary progressive aphasia. 10.1016/j.neubiorev.2019.02.020 [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, & McHugh PR (1975). “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research, 12(3), 189–198. 10.1016/0022-3956(75)90026-6 [DOI] [PubMed] [Google Scholar]
- Fredrickson BL (1998). What good are positive emotions? Review of General Psychology, 2(3), 300–319. 10.1037/1089-2680.2.3.300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredrickson BL (2013). Positive Emotions Broaden and Build. In Advances in Experimental Social Psychology (Vol. 47, pp. 1–53). Academic Press Inc. 10.1016/B978-0-12-407236-7.00001-2 [DOI] [Google Scholar]
- Gainotti G (1972). Emotional Behavior and Hemispheric Side of the Lesion. Cortex, 8(1), 41–55. 10.1016/S0010-9452(72)80026-1 [DOI] [PubMed] [Google Scholar]
- Gainotti G (2019). Emotions and the Right Hemisphere: Can New Data Clarify Old Models? In Neuroscientist (Vol. 25, Issue 3, pp. 258–270). SAGE Publications Inc. 10.1177/1073858418785342 [DOI] [PubMed] [Google Scholar]
- Gilbert KE, Nolen-Hoeksema S, & Gruber J (2013). Positive emotion dysregulation across mood disorders: How amplifying versus dampening predicts emotional reactivity and illness course. Behaviour Research and Therapy, 51(11), 736–741. 10.1016/j.brat.2013.08.004 [DOI] [PubMed] [Google Scholar]
- Gobbini M, Leibenluft E, Santiago N, & Haxby JV (2004). Social and emotional attachment in the neural representation of faces. NeuroImage, 22(4), 1628–1635. 10.1016/j.neuroimage.2004.03.049 [DOI] [PubMed] [Google Scholar]
- Gorno-Tempini MLL, Hillis AE, Weintraub S, Kertesz A, Mendez M, Cappa SF, Ogar JM, Rohrer JD, Black S, Boeve BF, Manes F, Dronkers NF, Vandenberghe R, Rascovsky K, Patterson K, Miller BL, Knopman DS, Hodges JR, Mesulam MM, & Grossman M (2011). Classification of primary progressive aphasia and its variants. Neurology, 76(11), 1006–1014. 10.1212/WNL.0b013e31821103e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber J (2011). Can feeling too good be bad? Positive emotion persistence (PEP) in bipolar disorder. In Current Directions in Psychological Science (Vol. 20, Issue 4, pp. 217–221). 10.1177/0963721411414632 [DOI] [Google Scholar]
- Gruber J, & Purcell J (2015). Positive Emotion Disturbance. In Emerging Trends in the Social and Behavioral Sciences (pp. 1–12). John Wiley & Sons, Inc. 10.1002/9781118900772.etrds0354 [DOI] [Google Scholar]
- Guo CC, Gorno-Tempini ML, Gesierich B, Henry M, Trujillo A, Shany-Ur T, Jovicich J, Robinson SD, Kramer JH, Rankin KP, Miller BL, & Seeley WW (2013). Anterior temporal lobe degeneration produces widespread network-driven dysfunction. Brain, 136(10), 2979–2991. 10.1093/brain/awt222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haxby JV, Hoffman EA, & Gobbini MI (2000). The distributed human neural system for face perception. In Trends in Cognitive Sciences (Vol. 4, Issue 6, pp. 223–233). 10.1016/S1364-6613(00)01482-0 [DOI] [PubMed] [Google Scholar]
- Hechtman LA, Raila H, Chiao JY, & Gruber J (2013). Positive Emotion Regulation and Psychopathology: A Transdiagnostic Cultural Neuroscience Approach. Journal of Experimental Psychopathology, 4(5), 502–528. 10.5127/jep.030412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua AY, Chen K-H, Brown CL, Lwi SJ, Casey JJ, Rosen HJ, Miller BL, & Levenson RW (2020). Physiological, behavioral and subjective sadness reactivity in frontotemporal dementia subtypes. Social Cognitive and Affective Neuroscience, 2020, 1–13. 10.1093/scan/nsaa007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua AY, Sible IJ, Perry DC, Rankin KP, Kramer JH, Miller BL, Rosen HJ, & Sturm VE (2018). Enhanced Positive Emotional Reactivity Undermines Empathy in Behavioral Variant Frontotemporal Dementia. Frontiers in Neurology, 9(JUN), 402. 10.3389/fneur.2018.00402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irish M, Hodges JR, & Piguet O (2014). Right anterior temporal lobe dysfunction underlies theory of mind impairments in semantic dementia. Brain : A Journal of Neurology, 137(Pt 4), 1241–1253. 10.1093/brain/awu003 [DOI] [PubMed] [Google Scholar]
- Irish M, Kumfor F, Hodges JR, & Piguet O (2013). A tale of two hemispheres: Contrasting socioemotional dysfunction in right- versus left-lateralised semantic dementia. Dementia e Neuropsychologia, 7(1), 88–95. 10.1590/S1980-57642013DN70100014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamath V, Chaney GAS, Deright J, & Onyike CU (2018). A meta-analysis of neuropsychological, social cognitive, and olfactory functioning in the behavioral and language variants of frontotemporal dementia. Psychological Medicine. 10.1017/S0033291718003604 [DOI] [PubMed] [Google Scholar]
- Kiernan JA (2012). Anatomy of the Temporal Lobe. Epilepsy Research and Treatment, 2012, 1–12. 10.1155/2012/176157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohn N, Eickhoff SB, Scheller M, Laird AR, Fox PT, & Habel U (2014). Neural network of cognitive emotion regulation - An ALE meta-analysis and MACM analysis. NeuroImage, 87, 345–355. 10.1016/j.neuroimage.2013.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer JH, Jurik J, Sha SJ, Rankin KP, Rosen HJ, Johnson JK, & Miller BL (2003). Distinctive Neuropsychological Patterns in Frontotemporal Dementia, Semantic Dementia, And Alzheimer Disease. Cognitive and Behavioral Neurology, 16(4), 211–218. 10.1097/00146965-200312000-00002 [DOI] [PubMed] [Google Scholar]
- Kumfor F, Hazelton JL, Rushby JA, Hodges JR, & Piguet O (2019). Facial expressiveness and physiological arousal in frontotemporal dementia: Phenotypic clinical profiles and neural correlates. Cognitive, Affective and Behavioral Neuroscience, 19(1), 197–210. 10.3758/s13415-018-00658-z [DOI] [PubMed] [Google Scholar]
- Kumfor F, & Piguet O (2012). Disturbance of emotion processing in frontotemporal dementia: A synthesis of cognitive and neuroimaging findings. Neuropsychology Review, 22(3), 280–297. 10.1007/s11065-012-9201-6 [DOI] [PubMed] [Google Scholar]
- Lambon Ralph MA, Cipolotti L, Manes F, & Patterson K (2010). Taking both sides: Do unilateral anterior temporal lobe lesions disrupt semantic memory? Brain, 133(11), 3243–3255. 10.1093/brain/awq264 [DOI] [PubMed] [Google Scholar]
- Landin-Romero R, Tan R, Hodges JR, & Kumfor F (2016). An update on semantic dementia: genetics, imaging, and pathology. Alzheimer’s Research & Therapy, 8(1), 52. 10.1186/s13195-016-0219-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levenson RW (2019). The Oxford Handbook of Positive Emotion and Psychopathology. The Oxford Handbook of Positive Emotion and Psychopathology. 10.1093/oxfordhb/9780190653200.001.0001 [DOI] [Google Scholar]
- Levenson RW (2003). Blood, Sweat, and Fears: The Autonomic Architecture of Emotion. Annals of the New York Academy of Sciences, 1000(1), 348–366. 10.1196/annals.1280.016 [DOI] [PubMed] [Google Scholar]
- Levenson RW, Sturm VE, & Haase CM (2014). Emotional and behavioral symptoms in neurodegenerative disease: a model for studying the neural bases of psychopathology. Annual Review of Clinical Psychology, 10(February), 581–606. 10.1146/annurev-clinpsy-032813-153653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman MD, Eisenberger NI, Crockett MJ, Tom SM, Pfeifer JH, & Way BM (2007). Putting feelings into words: Affect labeling disrupts amygdala activity in response to affective stimuli: Research article. Psychological Science, 18(5), 421–428. 10.1111/j.1467-9280.2007.01916.x [DOI] [PubMed] [Google Scholar]
- Lieberman MD, Inagaki TK, Tabibnia G, & Crockett MJ (2011). Subjective Responses to Emotional Stimuli During Labeling, Reappraisal, and Distraction. Emotion, 11(3), 468–480. 10.1037/a0023503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist KA, Gendron M, Barrett LF, & Dickerson BC (2014). Emotion perception, but not affect perception, is impaired with semantic memory loss. Emotion, 14(2), 375–387. 10.1037/a0035293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macoir J, Hudon C, Tremblay M-P, Laforce RJ, & Wilson MA (2019). The contribution of semantic memory to the recognition of basic emotions and emotional valence: Evidence from the semantic variant of primary progressive aphasia. Social Neuroscience, 14(6), 705–716. 10.1080/17470919.2019.1577295 [DOI] [PubMed] [Google Scholar]
- Margulies DS, & Petrides M (2013). Distinct parietal and temporal connectivity profiles of ventrolateral frontal areas involved in language production. Journal of Neuroscience, 33(42), 16846–16852. 10.1523/JNEUROSCI.2259-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall CR, Hardy CJD, Russell LL, Clark CN, Bond RL, Dick KM, Brotherhood E. v., Mummery CJ, Schott JM, Rohrer JD, Kilner JM, & Warren JD (2018). Motor signatures of emotional reactivity in frontotemporal dementia. Scientific Reports 2018 8:1, 8(1), 1–13. 10.1038/s41598-018-19528-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauss IB, McCarter L, Levenson RW, Wilhelm FH, & Gross JJ (2005). The tie that binds? Coherence among emotion experience, behavior, and physiology. Emotion, 5(2), 175–190. 10.1037/1528-3542.5.2.175 [DOI] [PubMed] [Google Scholar]
- McRae K, Misra S, Prasad AK, Pereira SC, & Gross JJ (2012). Bottom-up and top-down emotion generation: implications for emotion regulation. Social Cognitive and Affective Neuroscience, 7(3), 253–262. 10.1093/scan/nsq103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez MF, Mcmurtray A, Chen AK, Shapira JS, Mishkin F, Mendez MF, & Miller BL (2006). Functional neuroimaging and presenting psychiatric features in frontotemporal dementia. 77(1), 4–7. 10.1136/jnnp.2005.072496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam M, Wieneke C, Rogalski E, Cobia D, Thompson C, & Weintraub S (2009). Quantitative template for subtyping primary progressive aphasia. Archives of Neurology, 66(12), 1545–1551. 10.1001/archneurol.2009.288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mion M, Patterson K, Acosta-Cabronero J, Pengas G, Izquierdo-Garcia D, Hong YT, Fryer TD, Williams GB, Hodges JR, & Nestor PJ (2010). What the left and right anterior fusiform gyri tell us about semantic memory. Brain, 133(11), 3256–3268. 10.1093/brain/awq272 [DOI] [PubMed] [Google Scholar]
- Morris JC (1993). The clinical dementia rating (cdr): Current version and scoring rules. Neurology, 43(11), 2412–2414. 10.1212/wnl.43.11.2412-a [DOI] [PubMed] [Google Scholar]
- Morris JC (1997). Clinical Dementia Rating: A reliable and valid diagnostic and staging measure for dementia of the Alzheimer type. International Psychogeriatrics, 9(SUPPL. 1), 173–176. 10.1017/S1041610297004870 [DOI] [PubMed] [Google Scholar]
- Muhtadie L, Haase CM, Verstaen A, Sturm VE, Miller BL, & Levenson RW (2019). Neuroanatomy of Expressive Suppression: The Role of the Insula. Emotion. 10.1037/emo0000710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller V, Cieslik E, Turetsky B, & Eickhoff S (2012). Modeling crossmodal interactions in emotional audiovisual integration. Klinische Neurophysiologie, 43(01). 10.1055/s-0032-1301610 [DOI] [Google Scholar]
- Ochsner KN, & Gross JJ (2005). The cognitive control of emotion. Trends in Cognitive Sciences, 9(5), 242–249. 10.1016/j.tics.2005.03.010 [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Ray RR, Hughes B, Mcrae K, Cooper JC, Weber J, Gabrieli JDE, & Gross JJ (2009a). Bottom up and top down processes in emotion generation: common and distinct neural mechanisms. Psychol Sci, 20(11), 1322–1331. 10.1111/j.1467-9280.2009.02459.x.Bottom-Up [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner KN, Ray RR, Hughes B, Mcrae K, Cooper JC, Weber J, Gabrieli JDE, & Gross JJ (2009b). Bottom-Up and Top-Down Processes in Emotion Generation Common and Distinct Neural Mechanisms. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor CM, Clemson L, Flanagan E, Kaizik C, Brodaty H, Hodges JR, Piguet O, & Mioshi E (2016). The Relationship between Behavioural Changes, Cognitive Symptoms, and Functional Disability in Primary Progressive Aphasia: A Longitudinal Study. Dementia and Geriatric Cognitive Disorders, 42(3–4), 215–226. 10.1159/000449283 [DOI] [PubMed] [Google Scholar]
- Ogar JM, Baldo JV, Wilson SM, Brambati SM, Miller BL, Dronkers NF, & Gorno-Tempini ML (2011). Semantic dementia and persisting Wernicke’s aphasia: Linguistic and anatomical profiles. Brain and Language, 117(1), 28–33. 10.1016/j.bandl.2010.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Öngür D, Ferry AT, & Price JL (2003). Architectonic subdivision of the human orbital and medial prefrontal cortex. The Journal of Comparative Neurology, 460(3), 425–449. 10.1002/cne.10609 [DOI] [PubMed] [Google Scholar]
- Perry DC, Brown JA, Possin KL, Datta S, Trujillo A, Radke A, Karydas A, Kornak J, Sias AC, Rabinovici GD, Gorno-Tempini ML, Boxer AL, de May M, Rankin KP, Sturm VE, Lee SE, Matthews BR, Kao AW, Vossel KA, … Seeley WW (2017). Clinicopathological correlations in behavioural variant frontotemporal dementia. Brain, 140(12), 3329–3345. 10.1093/brain/awx254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry DC, Sturm VE, Seeley WW, Miller BL, Kramer JH, & Rosen HJ (2014). Anatomical correlates of reward-seeking behaviours in behavioural variant frontotemporal dementia. Brain, 137(6), 1621–1626. 10.1093/brain/awu075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrides M, Cadoret G, & Mackey S (2005). Orofacial somatomotor responses in the macaque monkey homologue of Broca’s area. Nature, 435(7046), 1235–1238. 10.1038/nature03628 [DOI] [PubMed] [Google Scholar]
- Petrides M, & Pandya DN (1988). Association fiber pathways to the frontal cortex from the superior temporal region in the rhesus monkey. Journal of Comparative Neurology, 273(1), 52–66. 10.1002/cne.902730106 [DOI] [PubMed] [Google Scholar]
- Petrides M, & Pandya DN (2007). Behavioral/Systems/Cognitive Efferent Association Pathways from the Rostral Prefrontal Cortex in the Macaque Monkey. 10.1523/JNEUROSCI.2419-07.2007 [DOI] [PMC free article] [PubMed]
- Petrides M, & Pandya DN (2009a). Association Pathways of the Prefrontal Cortex and Functional Observations. In Principles of Frontal Lobe Function. Oxford University Press. 10.1093/acprof:oso/9780195134971.003.0003 [DOI] [Google Scholar]
- Petrides M, & Pandya DN (2009b). Distinct parietal and temporal pathways to the homologues of Broca’s area in the monkey. PLoS Biology, 7(8). 10.1371/journal.pbio.1000170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picó-Pérez M, Alonso P, Contreras-Rodríguez O, Martínez-Zalacaín I, López-Solà C, Jiménez-Murcia S, Verdejo-García A, Menchón JM, & Soriano-Mas C (2018). Dispositional use of emotion regulation strategies and resting-state cortico-limbic functional connectivity. Brain Imaging and Behavior, 12(4), 1022–1031. 10.1007/s11682-017-9762-3 [DOI] [PubMed] [Google Scholar]
- Pressman PS, Simpson M, Gola K, Shdo SM, Spinelli EG, Miller BL, Gorno-Tempini ML, Rankin K, & Levenson RW (2017). Observing conversational laughter in frontotemporal dementia. Journal of Neurology, Neurosurgery & Psychiatry, 88(5), 418–424. 10.1136/jnnp-2016-314931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price CJ (2010). The anatomy of language: A review of 100 fMRI studies published in 2009. In Annals of the New York Academy of Sciences (Vol. 1191, pp. 62–88). Blackwell Publishing Inc. 10.1111/j.1749-6632.2010.05444.x [DOI] [PubMed] [Google Scholar]
- Rankin KP, Gorno-Tempini ML, Allison SC, Stanley CM, Glenn S, Weiner MW, & Miller BL (2006). Structural anatomy of empathy in neurodegenerative disease. Brain, 129(11), 2945–2956. 10.1093/brain/awl254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rascovsky K, Hodges JR, Knopman D, Mendez MF, Kramer JH, Neuhaus J, Van Swieten JC, Seelaar H, Dopper EGPP, Onyike CU, Hillis AE, Josephs KA, Boeve BF, Kertesz A, Seeley WW, Rankin KP, Johnson JK, Gorno-Tempini ML, Rosen H, … Miller BL (2011). Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain, 134(9), 2456–2477. 10.1093/brain/awr179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen HJ, Gorno-Tempini ML, Goldman WP, Perry RJ, Schuff N, Weiner M, Feiwell R, Kramer JH, & Miller BL (2002). Patterns of brain atrophy in frontotemporal dementia and semantic dementia. Neurology, 58(2), 198–208. 10.1212/WNL.58.2.198 [DOI] [PubMed] [Google Scholar]
- Saper CB (2002). The Central Autonomic Nervous System: Conscious Visceral Perception and Autonomic Pattern Generation. Annual Review of Neuroscience, 25(1), 433–469. 10.1146/annurev.neuro.25.032502.111311 [DOI] [PubMed] [Google Scholar]
- Seeley WW, Bauer A, Miller B, Gorno-Tempini ML, Kramer JH, Weiner M, & Rosen HJ (2005). The natural history of temporal variant frontotemporal dementia. Neurology, 64(8), 1384–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW, Crawford R, Rascovsky K, Kramer JH, Weiner M, Miller BL, & Gorno-Tempini ML (2008). Frontal paralimbic network atrophy in very mild behavioral variant frontotemporal dementia. Archives of Neurology, 65(2), 249–255. 10.1001/archneurol.2007.38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW, Crawford R, Zhou J, Miller B, & Greicius M (2009). Neurodegenerative Diseases Target Large-Scale Human Brain Networks. Neuron, 62(1), 42–52. 10.1016/j.neuron.2009.03.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shdo SM, Ranasinghe KG, Gola KA, Mielke CJ, Sukhanov PV, Miller BL, & Rankin KP (2016). Deconstructing empathy: Neuroanatomical dissociations between affect sharing and prosocial motivation using a patient lesion model. Neuropsychologia, September 2016, 1–10. 10.1016/j.neuropsychologia.2017.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu H, Komori K, Fukuhara R, Shinagawa S, Toyota Y, Kashibayashi T, Sonobe N, Matsumoto T, Mori T, Ishikawa T, Hokoishi K, Tanimukai S, Ueno S-I, & Ikeda M (2011). Clinical profiles of late-onset semantic dementia, compared with early-onset semantic dementia and late-onset Alzheimer’s diseasep syg_351 46..53. 10.1111/j.1479-8301.2010.00351.x [DOI] [PubMed]
- Shiota MN, Neufeld SL, Yeung WH, Moser SE, & Perea EF (2011). Feeling Good: Autonomic Nervous System Responding in Five Positive Emotions. Emotion, 11(6), 1368–1378. 10.1037/a0024278 [DOI] [PubMed] [Google Scholar]
- Silvers JA, Buhle JT, Ochsner KN, & Dean J (2012). The neuroscience of emotion regulation: basic mechanisms and their role in development, aging, and psychopathology. Neuroscience of Emotion Regulation. [Google Scholar]
- Snowden JS, Bathgate D, Varma AR, Blackshaw A, Gibbons ZC, & Neary D (2001). Distinct behavioural profiles in frontotemporal dementia and semantic dementia. Journal of Neurology, Neurosurgery, and Psychiatry, 70(3), 323–332. 10.1136/jnnp.70.3.323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturm VE, Ascher EA, Miller BL, & Levenson RW (2008). Diminished Self-Conscious Emotional Responding in Frontotemporal Lobar Degeneration Patients. Emotion, 8(6), 861–869. 10.1037/a0013765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturm VE, McCarthy ME, Yun I, Madan A, Yuan JW, Holley SR, Ascher EA, Boxer AL, Miller BL, & Levenson RW (2011). Mutual gaze in Alzheimer’s disease, frontotemporal and semantic dementia couples. Social Cognitive and Affective Neuroscience, 6(3), 359–367. 10.1093/scan/nsq055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturm VE, Sollberger M, Seeley WW, Rankin KP, Ascher EA, Rosen HJ, Miller BL, & Levenson RW (2013). Role of right pregenual anterior cingulate cortex in self-conscious emotional reactivity. Social Cognitive and Affective Neuroscience, 8(4), 468–474. 10.1093/scan/nss023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturm VE, Yokoyama JS, Eckart JA, Zakrzewski J, Rosen HJ, Miller BL, Seeley WW, & Levenson RW (2015). Damage to left frontal regulatory circuits produces greater positive emotional reactivity in frontotemporal dementia. Cortex, 64(415), 55–67. 10.1016/j.cortex.2014.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda A, Sturm VE, Rankin KP, Ketelle R, Miller BL, & Perry DC (2019). Relationship Turmoil and Emotional Empathy in Frontotemporal Dementia. Alzheimer Disease and Associated Disorders, 33(3), 260–265. 10.1097/WAD.0000000000000317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson SA, Patterson K, & Hodges JR (2003). Left/right asymmetry of atrophy in semantic dementia: Behavioral-cognitive implications. Neurology, 61(9), 1196–1203. 10.1212/01.WNL.0000091868.28557.B8 [DOI] [PubMed] [Google Scholar]
- Verstaen A, Eckart JA, Muhtadie L, Otero MC, Sturm VE, Haase CM, Miller BL, & Levenson RW (2016). Insular atrophy and diminished disgust reactivity. Emotion, 16(6), 903–912. 10.1037/emo0000195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SM, Henry ML, Besbris M, Ogar JM, Dronkers NF, Jarrold W, Miller BL, & Gorno-Tempini ML (2010). Connected speech production in three variants of primary progressive aphasia. Brain, 133(7), 2069–2088. 10.1093/BRAIN/AWQ129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley JD, Khan BK, Murthy NK, Miller BL, & Rankin KP (2011). The diagnostic challenge of psychiatric symptoms in neurodegenerative disease: Rates of and risk factors for prior psychiatric diagnosis in patients with early neurodegenerative disease. Journal of Clinical Psychiatry, 72(2), 126–133. 10.4088/JCP.10m06382oli [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data generated by the UCSF Memory and Aging Center are available upon request. All data requests can be submitted through the UCSF Memory and Aging Center Resource Request Form (http://memory.ucsf.edu/resources/data). Academic, not-for-profit investigators with Institutional Review Board approval from the UCSF Human Research Protection Program can request data for research studies. The UCSF Human Research Protection Program will not review the application until the UCSF Memory and Aging Center Executive Committee has signed off on the proposal and consent form. Data are not publicly available because they contain information that could compromise the privacy of the participants.


