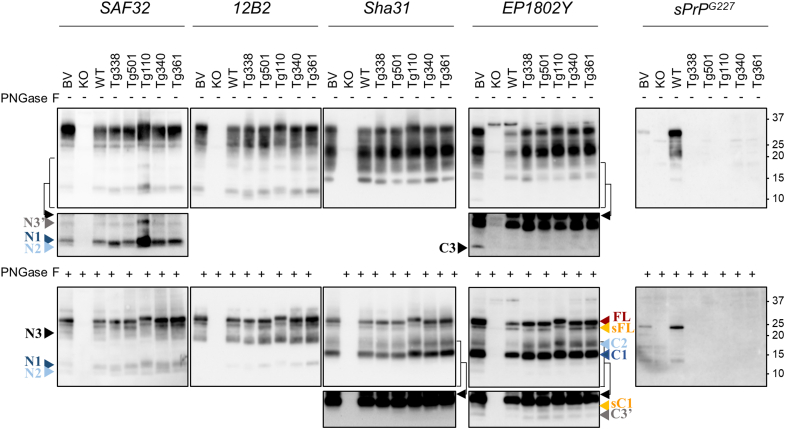
Figure 3.
Comparison of PrPCproteoforms present in the brain ofwild typeand transgenic rodent models. Representative western blots of brain homogenates prepared from bank vole, PrP-KO mouse (KO), wild type mouse (WT), tg338 (expressing sheep-VRQ PrPC), tg501 (expressing sheep/goat-ARQ PrPC), tg110 (expressing bovine PrPC), tg340 and tg361 (expressing human M129 and human V129 PrPC, respectively). All samples were left untreated (“−,” upper blots of the panel) or subjected to PNGase F treatment (“+,” lower blots of the panel). Replica blots were probed with different antibodies, indicated at the top of each pair. PrPC proteoforms are indicated with arrowhead colored differently for different proteolytic events: orange is used for shedding (sFL and sC1), blue and light blue for α- (N1/C1) and β-cleavages (N2/C2), and gray and black for γ-cleavages (N3/C3 and N3′/C3′). A red arrowhead indicates FL-PrPC. Note that the overall PrPC proteoform pattern is very similar among the rodent models, apart from the apparent molecular weight (MW) of FL, sFL, C2, N1, and N3, which is higher in tg110 than in other models because of an extra octarepeat in bovine PrPC. As expected because of sequence alterations, sFL was detected in none of the transgenic mouse lines by the mouse sPrP-specific antibody (upper and lower blots on the right, orange arrowhead). Tissue equivalents (TEs) loaded per lane were 0.2 and 0.06 mg for untreated (−) and PNGase F-treated (+) samples. The positions of MW markers (and the respective kilodaltons) are reported on the right. A long exposure (black arrow, the bracket indicates the portion of the blot shown after a longer exposure) was necessary for a clearer identification of less abundant PrPC fragments. PrPC, cellular prion protein; sC1, shed C1; sFL, shed FL-PrPC.