Summary
Vaccine effectiveness of BNT162b2 and CoronaVac against COVID-19-associated hospitalization and moderate-to-severe disease due to SARS-CoV-2 Omicron BA.2 is studied from the 1.36 million doses administered to 766,601 of 953,400 children aged 3–11 years and adolescents aged 12–18 years in Hong Kong as of April 2022. These vaccines confer substantial protection.
Graphical abstract
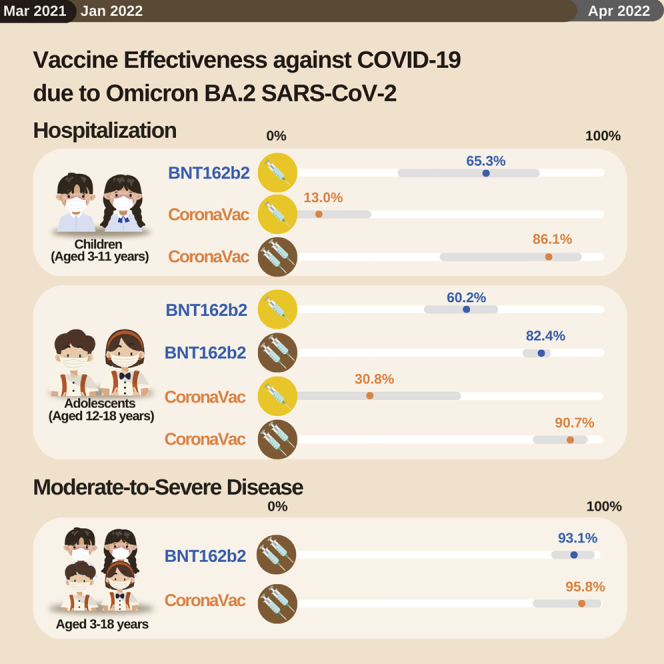
Vaccine effectiveness of BNT162b2 and CoronaVac against COVID-19-associated hospitalization and moderate-to-severe disease due to SARS-CoV-2 Omicron BA.2 is studied from the 1.36 million doses administered to 766,601 of 953,400 children aged 3–11 years and adolescents aged 12–18 years in Hong Kong as of April 2022. These vaccines confer substantial protection.
Main text
The SARS-CoV-2 Omicron variant continues to cause millions of COVID-19-associated pediatric hospitalizations, severe disease, and death globally.1 BNT162b2 and CoronaVac are among the top most widely used COVID-19 vaccines across the world. In Hong Kong (HK), these two vaccines have been authorized for adolescents since March 16, 2021 and children since January 21, 2022, while the third dose of both vaccines for all healthy adolescents and children commenced on March 11, 2022 and April 14, 2022, respectively (Figure S1). Consequently, 1.36 million vaccine doses had been administered to 766,601 (80.4%) of the total HK pediatric population of 953,400 from March 2021 to April 2022, with 331,948 (65.6%) of 506,100 being children aged 3–11 years (aged 5–11 for BNT162b2 and 3–11 years for CoronaVac) and 434,653 (97.1%) of 447,300 being adolescents aged 12–18 years.2 Of 258,466 children, 73,482 had received at least one dose of BNT162b2 (22.1% among those vaccinated) or CoronaVac (77.9%), respectively, while 174,152 (34.4% of the total HK pediatric population) remained unvaccinated (Figure S2). For adolescents, 372,753 and 61,900 had received at least one dose of BNT162b2 (85.8%) or CoronaVac (14.2%), respectively, whereas 12,627 (2.8%) remained unvaccinated.
The early phases of such a widespread vaccination program relied on data from licensing trials that demonstrated non-inferior neutralization and efficacy against COVID-19 for BNT162b2 in those aged 5–17 and immunogenicity for CoronaVac in those aged 3–17 but not effectiveness against hospitalization or severe disease, despite these endpoints being the most relevant real-life outcomes. Evaluation of real-life vaccine effectiveness (VE) against hospitalization and severe disease in children and adolescents is important because they have different immune responses to vaccines than adults do.3 VE studies performed in children and adolescents during Omicron BA.1 waves revealed lower VE against symptomatic COVID-19 and generally preserved VE against severe outcomes,4,5,6,7,8 yet isolated studies showed low VE against hospitalization or severe outcomes.4,9 Moreover, pediatric VE data on the subvariant BA.2, which is antigenically distinct from BA.1, are lacking. A major Omicron wave predominated by the highly transmissible BA.2 in HK caused many pediatric hospitalizations, clinical complications, and deaths in early 2022 despite the continuation of the elimination strategy that had suppressed SARS-CoV-2 circulation in the past.1,2
During this BA.2 wave, almost all hospitalized pediatric COVID-19 cases received medical care in public hospitals, all of which utilize the same electronic health software operated by the HK Hospital Authority (HA). The HK Department of Health (DH) had instituted a rigorous tracking system on COVID-19 vaccinations and compulsory reporting of infections. These recent events and the established infrastructure that maintains such a robust archive of data have created the possibility to study VE against severe disease by BA.2 in a population unexposed to prior SARS-CoV-2 variants.1
By retrieving population-level vaccine coverage statistics from DH and clinical data of hospitalized pediatric patients with laboratory-confirmed COVID-19 from HA, we aimed to determine the VE against COVID-19-associated hospitalization and moderate-to-severe disease by BNT162b2 in children and adolescents aged 5–18 years and CoronaVac in children and adolescents aged 3–18 years during the January 2022 to April 2022 BA.2 wave using an ecological study design. In addition, based on the VE results, we investigated the whole population impact of vaccination, with estimations of cases of COVID-19-associated hospitalization and moderate-to-severe disease averted by BNT162b2 and CoronaVac in children and adolescents.
During the study period of January 2022 (January 21, 2022 for children aged 3–11 years and January 1, 2022 for adolescents aged 12–18 years) to April 19, 2022, there were 1,099 and 455 COVID-19-associated hospitalizations for children and adolescents, respectively (Table S1). Over this study period, the daily numbers of COVID-19-associated hospitalizations were relatively higher for children (Figure S3A) than adolescents (Figure S3B), which peaked in late January to February 2022 for both age groups. A similar pattern was observed for the daily numbers of COVID-19-associated moderate-to-severe disease, but the peak occurred later in late February 2022 (Figures S3C and Figure S3D). 197 children and 31 adolescents had COVID-19-associated moderate-to-severe disease (Table S1). There were few patients with comorbidities (Table S2).
For VE against COVID-19-associated hospitalization, we found some protection from 1 dose of BNT162b2 in children (65.6%, 95% CI 38.2–82.5, p = 0.0008) and adolescents (60.2%, 95% CI 47.0–70.2, p < 0.0001) (Table 1). Two doses of CoronaVac for both children (86.2%, 95% CI 65.8–95.9, p = 0.0001) and adolescents (90.7%, 95% CI 79.2–96.8, p < 0.0001) and 2 doses of BNT162b for adolescents (82.3%, 95% CI 76.9–86.4, p < 0.0001) conferred high protection. VE was unable to be estimated for 2 doses of BNT162b2 in children because the second dose in this population began at the end of the study period (BNT162b2 began on February 16, 2022 in children and the second dose was recommended for 8 weeks later, which was April 13, 2022). There was no COVID-19-associated hospitalization or moderate-to-severe disease for any of those who received 3 doses of BNT162b2 (2 children and 61,237 adolescents) or CoronaVac (40 children and 7,286 adolescents). For COVID-19-associated moderate-to-severe disease, 1 dose of BNT162b2 (84.6%, 95% CI 69.7–93.2, p < 0.0001) and 2 doses of either BNT162b2 (93.1%, 95% CI 86.4–97.0, p < 0.0001) or CoronaVac (95.8%, 95% CI 80.7–99.8, p = 0.0017) conferred high protection. Results from the sensitivity analyses were similar (Table S3; Table S4).
Table 1.
Vaccine effectiveness against COVID-19-associated hospitalization and moderate-to-severe disease with adjustment by calendar days and 14-day lag in children (aged 3–11 years, January 21, 2022 to April 19, 2022) and adolescents (aged 12–18 years, January 1, 2022 to April 19, 2022)
| Numbers of cases | Mean days elapsed since the last dose (SD) | VE estimate | 95%CI | p value | |
|---|---|---|---|---|---|
| VE against hospitalization | |||||
| Children (aged 3–11 years) | |||||
| BNT162b2, 1 dose | 12 | 27.4 (14.7) | 65.6% | 38.2–82.5 | 0.0008 |
| CoronaVac, 1 dose | 127 | 23.8 (7.5) | 13.5% | −14.0–34.6 | 0.3187 |
| CoronaVac, 2 doses | 4 | 23.3 (10.7) | 86.2% | 65.8–95.9 | 0.0001 |
| Unvaccinated | 956 | Ref. | Ref. | Ref. | Ref. |
| Adolescents (aged 12–18 years) | |||||
| BNT162b2, 1 dose | 114 | 132.2 (54.1) | 60.2% | 47.0–70.2 | <0.0001 |
| CoronaVac, 1 dose | 22 | 34.7 (16.0) | 31.0% | −9.34–58.3 | 0.1286 |
| BNT162b2, 2 doses | 165 | 171.5 (51.2) | 82.3% | 76.9–86.4 | <0.0001 |
| CoronaVac, 2 doses | 5 | 47.4 (19.6) | 90.7% | 79.2–96.8 | <0.0001 |
| Unvaccinated | 149 | Ref. | Ref. | Ref. | Ref. |
| VE against moderate-to-severe disease | |||||
| Children and adolescents (aged 3–18 years) | |||||
| BNT162b2, 1 dose | 8 | 126.1 (54.0) | 84.6% | 69.7–93.2 | <0.0001 |
| CoronaVac, 1 dose | 33 | 22.2 (5.7) | 33.7% | −4.6–58.9 | 0.0855 |
| BNT162b2, 2 doses | 8 | 185.6 (49.4) | 93.1% | 86.4–97.0 | <0.0001 |
| CoronaVac, 2 doses | 1 | 39.0 (0.0) | 95.8% | 80.7–99.8 | 0.0017 |
| Unvaccinated | 178 | Ref. | Ref. | Ref. | Ref. |
SD, standard deviation; VE, vaccine effectiveness; CI, confidence interval; Ref. reference
The observed (actual) and expected numbers of COVID-19-associated hospitalization in the absence of vaccination were 1,099 and 1,167 (95% CI 1,111–1,240) for children and 455 and 1,454 (95% CI 908–2,111) for adolescents, respectively. Hence, 68 (95% CI 12–141) and 999 (95% CI 453–1,656) cases of COVID-19-associated hospitalization were averted due to vaccination for children (Figure S4A) and adolescents (Figure S4B), respectively (see supplemental experimental procedures, related to Table S6 and Data S1).1,2,3,10,11 There were 197 and 31 cases of COVID-19-associated moderate-to-severe disease for children and adolescents, while the expected numbers were 242 (95% CI 205–244) and 178 (95% CI 93–384), respectively. The numbers of moderate-to-severe cases averted due to vaccination were 45 (95% CI 8–47) for children (Figure S4C) and 147 (95% CI 62–353) for adolescents (Figure S4D). The adjusted estimations of cases averted are depicted in Figure S5 (see supplemental experimental procedures).
While VE had been shown to be lower against hospitalization and severe COVID-19 due to Omicron BA.1, especially in adults, the current study demonstrates that for children and adolescents, BNT162b2 and CoronaVac continue to confer protection against COVID-19-associated hospitalization and moderate-to-severe disease. More cases of COVID-19-associated hospitalization and moderate-to-severe disease were averted by the vaccination program that began in 2021 for adolescents than vaccination that commenced during the middle of the study period in 2022 for children. VE could not be estimated for 3 doses of either vaccine because none of the 68,565 who received the third dose required hospitalization or had moderate-to-severe disease. Sensitivity analysis using 7-day lag showed roughly similar but higher VE estimates, especially for dose 1, possibly due to partial protection against severe outcomes by an early response that were classified as unvaccinated.
VE estimates against severe outcomes with Omicron BA.1 in children and adolescents have varied between studies performed in different settings. Most notably, Price and colleagues found 43% VE against COVID-19-associated hospitalizations 2–22 weeks after 2 doses of BNT162b2 in adolescents and 68% after 2 doses in children using a test-negative design in 31 pediatric hospitals in the United States.4 Sacco and colleagues in Italy found 41% VE against severe COVID-19 during BA.1 predominance by a population cohort analysis.9 Our study is unique as we analyze VE in a largely prior uninfected population, meaning our unvaccinated population will not be protected by a previous infection, for which it is difficult to be completely adjusted.1,2,3 Additionally, hospitalization as an outcome measure varies across different settings and is imperfect as muddled by persons with incidental infection.12 Our data support the high VE of BNT162b2 against severe COVID-19 in adolescents and children estimated by several other groups.5,13
CoronaVac is an inactivated whole-virus vaccine that has been associated with weaker neutralization titers yet comparable or higher T cell responses than mRNA and other vaccine platforms.3 In this analysis, we found high protection against hospitalization and moderate-to-severe disease after 2 doses in both adolescents and children. These results are in line with analyses in Chile, Brazil, and Argentina that inactivated COVID-19 vaccines elicit high protection against severe outcomes in children and adolescents.6,7,14 This is likely due to T cell responses elicited by the inactivated whole-virus vaccines, which are directed against all structural proteins of SARS-CoV-2 and not just the spike.3 We have previously shown preserved T cell responses against SARS-CoV-2 S, N, and M proteins for BA.1. The current findings from this study strongly support the use of CoronaVac in children and adolescents against severe COVID-19.
Overall, it can be concluded that the BNT162b2 or CoronaVac vaccines confer protection against Omicron BA.2-associated severe clinical outcomes for children and adolescents, with no apparent differences observed across the two vaccine types. Public advocacy and education are essential to promote immediate vaccination in children so these individuals can benefit from timely protection against COVID-19 and so that more severe cases within the whole population can be averted. Further research on the impact of the third dose and durability of these vaccines in children and adolescents, VE against multisystem inflammatory syndrome in children (MIS-C), and protection from vaccination in younger children ages 6 months to 2 years as the COVID-19 vaccines have become available for this age group recently is necessary and planned.
Numerous VE studies must be performed as these are conducted under variable settings, and the conclusions need to be interpreted in the context of all available literature, including and not solely on the current study results.15 This study has limitations, because research with observational VE designs, such as the current study, can be prone to bias, which can include the possibility of residual confounding unaccounted by other factors. As HK tended to hospitalize most diagnosed cases, especially those youngest and at the very beginning of the pandemic, VE against hospitalization may be lower and closer to VE against infection in our dataset. However, we also evaluated VE against moderate-to-severe COVID-19, which is stringent and revealed even higher VE. Due to insufficient power, we did not evaluate VE against progression from infection to hospitalization, moderate-to-severe disease, or death alone. Nevertheless, by using such big datasets that already included the most essential endpoints with an adequate sample size, we were able to reach clinically pertinent conclusions. Misclassification bias is possible because some deaths occurred before hospitalization. International Classification of Diseases, Ninth Revision (ICD-9) coding was manually entered and could be erroneous as detailed clinical notes were not requested to verify the ICD-9 coding. Preexisting comorbidities could not be adjusted, though they are rare in the pediatric population and not expected to influence VE estimates significantly for children and adolescents. There were few numbers of unvaccinated adolescents, and thus there is the possibility that differences between the unvaccinated and vaccinated were not fully accounted for. We were unable to evaluate waning of VE over time because many children and adolescents were recently vaccinated or received boosters, but follow-up studies have been arranged. As the HK population had very low levels of circulation of prior variants, the current findings are more applicable to parts of the world that had low past exposures. The person-time under 14 days of vaccination was categorized as the previous dose or unvaccinated <14 days after dose 1, which can underestimate the VE of the previous dose if there was exposure around the time of vaccination. Additionally, there is the possibility that there were different social-distancing practices or other risk behaviors in the unvaccinated compared with the vaccinated individuals, or vice versa, that could affect the VE. However, most individuals in HK maintained strict social-distancing practices, especially during the BA.2 wave regardless of vaccination status, and masking was mandatory in public.
Acknowledgments
We express our deepest gratitude to the health care workers who provided vaccination at the Community Vaccination Centres and clinical care to patients with COVID-19 in the hospitals. We are grateful to the Centre for Health Protection, Department of Health and the Hospital Authority, both of the Hong Kong Governments for providing these pertinent, pseudonymized datasets. We thank Dr. Minal K. Patel from the Department of Immunization, Vaccines, and Biologicals, World Health Organization, Geneva, Switzerland for her critical review, comments, and suggestions during the study planning and drafting of this manuscript. We are thankful to the Providence Foundation for supporting this study.
Author contributions
Y.L.L. conceptualized the study. W.H.S.W., Y.L.L., J.S.R.D., and D.L. designed the study. Y.L.L. led the acquisition of data and funding. Y.L.L. and W.H.S.W. supervised the project. W.H.S.W. provided software support. W.H.S.W., K.M.Y., H.-K.S., J.S.R.D., D.L., and Y.L.L. had unrestricted access to all data. K.M.Y., H.-K.S., and W.H.S.W. curated the data and estimated the VE. K.M.Y. and J.S.R.D. replicated and verified the software code output. W.H.S.W. oversaw the statistical analyses. J.S.R.D., D.L., K.M.Y., and D.H.L.L. visualized the data. J.S.R.D. and D.L. wrote the first draft of the manuscript, which was supervised, reviewed, and edited by Y.L.L. with input from W.H.S.W. All authors reviewed, approved, and agreed to take full responsibility of the content of the manuscript, the accuracy of its data, and fidelity of the statistical analysis.
Declaration of interests
Y.L.L. chairs the Scientific Committee on Vaccine Preventable Diseases of the HK Government.
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.xcrm.2023.100936.
Contributor Information
Wilfred H.S. Wong, Email: whswong@hku.hk.
Yu Lung Lau, Email: lauylung@hku.hk.
Supplemental information
Data availability
Individual-level data are unable to be shared due to third party use restrictions. The aggregate dataset is appended in Table S6, and the R code is available at https://github.com/kmanyip/BA2-VE-hosp-and-mod-sev and is included as Data S1.
References
- 1.Tso W.W.Y., Kwan M.Y.W., Wang Y.L., Leung L.K., Leung D., Chua G.T., Ip P., Fong D.Y.T., Wong W.H.S., Chan S.H.S., et al. Severity of SARS-CoV-2 Omicron BA. 2 infection in unvaccinated hospitalized children: comparison to influenza and parainfluenza infections. Emerg. Microbes Infect. 2022;11:1742–1750. doi: 10.1080/22221751.2022.2093135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Leung D., Rosa Duque J.S., Yip K.M., So H.K., Wong W.H.S., Lau Y.L. Effectiveness of BNT162b2 and CoronaVac in children and adolescents against SARS-CoV-2 infection during Omicron BA.2 wave in Hong Kong. Commun. Med. 2023;3:3. doi: 10.1038/s43856-022-00233-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rosa Duque J.S., Wang X., Leung D., Cheng S.M.S., Cohen C.A., Mu X., Hachim A., Zhang Y., Chan S.M., Chaothai S., et al. Immunogenicity and reactogenicity of SARS-CoV-2 vaccines BNT162b2 and CoronaVac in healthy adolescents. Nat. Commun. 2022;13:3700. doi: 10.1038/s41467-022-31485-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Price A.M., Olson S.M., Newhams M.M., Halasa N.B., Boom J.A., Sahni L.C., Pannaraj P.S., Irby K., Bline K.E., Maddux A.B., et al. BNT162b2 protection against the Omicron variant in children and adolescents. N. Engl. J. Med. 2022;386:1899–1909. doi: 10.1056/NEJMoa2202826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tan S.H.X., Cook A.R., Heng D., Ong B., Lye D.C., Tan K.B. Effectiveness of BNT162b2 vaccine against omicron in children 5 to 11 years of age. N. Engl. J. Med. 2022;387:525–532. doi: 10.1056/NEJMoa2203209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jara A., Undurraga E.A., Zubizarreta J.R., Gonzalez C., Acevedo J., Pizarro A., Vergara V., Soto-Marchant M., Gilabert R., Flores J.C., et al. Effectiveness of CoronaVac in children 3-5 years of age during the SARS-CoV-2 Omicron outbreak in Chile. Nat. Med. 2022;28:1377–1380. doi: 10.1038/s41591-022-01874-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Florentino P.T.V., Alves F.J.O., Cerqueira-Silva T., Oliveira V.d.A., Júnior J.B.S., Jantsch A.G., Penna G.O., Boaventura V., Werneck G.L., Rodrigues L.C., et al. Vaccine effectiveness of CoronaVac against COVID-19 among children in Brazil during the Omicron period. Nat. Commun. 2022;13:4756. doi: 10.1038/s41467-022-32524-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cohen-Stavi C.J., Magen O., Barda N., Yaron S., Peretz A., Netzer D., Giaquinto C., Judd A., Leibovici L., Hernán M.A., et al. BNT162b2 vaccine effectiveness against Omicron in children 5 to 11 years of age. N. Engl. J. Med. 2022;387:227–236. doi: 10.1056/NEJMoa2205011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sacco C., Del Manso M., Mateo-Urdiales A., Rota M.C., Petrone D., Riccardo F., Bella A., Siddu A., Battilomo S., Proietti V., et al. Effectiveness of BNT162b2 vaccine against SARS-CoV-2 infection and severe COVID-19 in children aged 5-11 years in Italy: a retrospective analysis of January-April, 2022. Lancet. 2022;400:97–103. doi: 10.1016/S0140-6736(22)01185-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.UK Health Security Agency . 2022. COVID-19 Vaccine Surveillance Report: Week 19.https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1075115/COVID-19_vaccine_surveillance_report_12_May_2022_week_19.pdf [Google Scholar]
- 11.Haas E.J., McLaughlin J.M., Khan F., Angulo F.J., Anis E., Lipsitch M., Singer S.R., Mircus G., Brooks N., Smaja M., et al. Infections, hospitalisations, and deaths averted via a nationwide vaccination campaign using the Pfizer–BioNTech BNT162b2 mRNA COVID-19 vaccine in Israel: a retrospective surveillance study. Lancet Infect. Dis. 2022;22:357–366. doi: 10.1016/s1473-3099(21)00566-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feikin D.R., Abu-Raddad L.J., Andrews N., Davies M.A., Higdon M.M., Orenstein W.A., Patel M.K. Assessing vaccine effectiveness against severe COVID-19 disease caused by omicron variant. Report from a meeting of the World Health Organization. Vaccine. 2022;40:3516–3527. doi: 10.1016/j.vaccine.2022.04.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fowlkes A.L., Yoon S.K., Lutrick K., Gwynn L., Burns J., Grant L., Phillips A.L., Ellingson K., Ferraris M.V., LeClair L.B., et al. Effectiveness of 2-dose BNT162b2 (Pfizer BioNTech) mRNA vaccine in preventing SARS-CoV-2 infection among children aged 5–11 years and adolescents aged 12–15 years — PROTECT cohort. MMWR Morb. Mortal. Wkly. Rep. 2022;71:422–428. doi: 10.15585/mmwr.mm7111e1. July 2021–February 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gonzalez S., Olszevicki S., Gaiano A., Baino A.N.V., Regairaz L., Salazar M., Pesci S., Marin L., Martinez V.V.G., Varela T., et al. Effectiveness of BBIBP-CorV, BNT162b2 and mRNA-1273 vaccines against hospitalisations among children and adolescents during the Omicron outbreak in Argentina: a retrospective cohort study. Lancet Reg. Health. Am. 2022;13 doi: 10.1016/j.lana.2022.100316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feikin D.R., Higdon M.M., Abu-Raddad L.J., Andrews N., Araos R., Goldberg Y., Groome M.J., Huppert A., O'Brien K.L., Smith P.G., et al. Duration of effectiveness of vaccines against SARS-CoV-2 infection and COVID-19 disease: results of a systematic review and meta-regression. Lancet. 2022;399:924–944. doi: 10.1016/s0140-6736(22)00152-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Individual-level data are unable to be shared due to third party use restrictions. The aggregate dataset is appended in Table S6, and the R code is available at https://github.com/kmanyip/BA2-VE-hosp-and-mod-sev and is included as Data S1.


