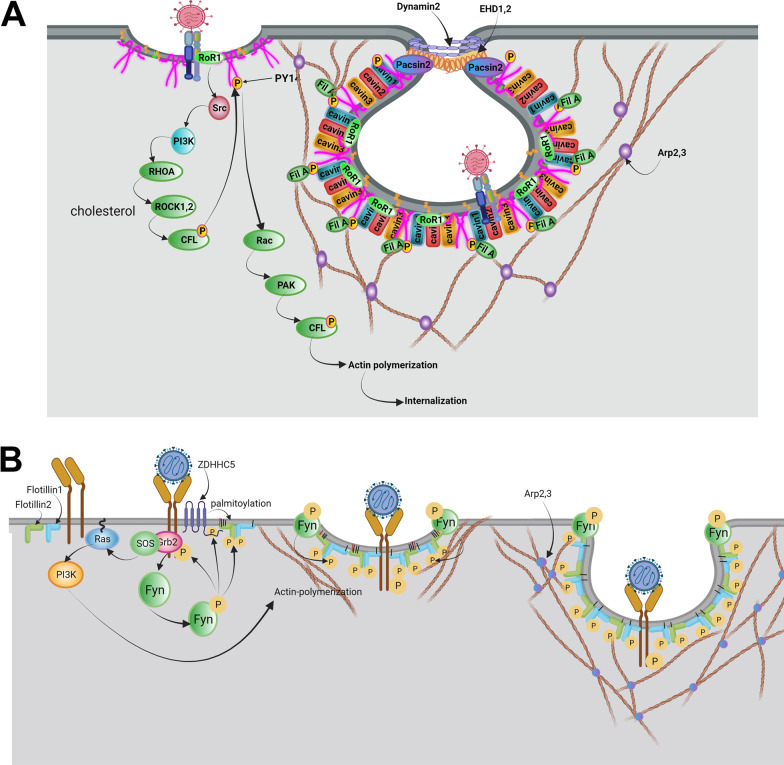
Fig. 6.
Internalization of the virus using Caveolae-dependent endocytosis (A). Attachment of virions to the membrane receptors in membrane microdomains enriched in cholesterol and PtdSer activates receptor tyrosine kinase-like orphan receptor 1 (ROR1) and subsequently directs activation of Src, PI3K, RhoA, ROCK, CFL1 signaling pathway, at the end leads phosphorylation of Caveolin1. ROR1, as a scaffold protein, facilitates the interaction of cavin-1 and phosphorylated Caveolin1 at the PM. Also, Caveolin1 recruits EHD1,2 and Dynamin2 via binding to the Pacsin2, thus leading to the formation of the caveolae neck and session of caveolae vesicles. On the other hand, phosphorylated Caveolin1 leads to actin polymerization via the activation of Rac1, PAK1, and CFL1 signaling pathways. Caveolin1 through binding to Filamin A is associated with actin proteins and regulates caveolae vesicle trafficking. Possible mechanism of virus entry mediated by flotillin-dependent endocytosis (B). Initial attachment of the virus to the cell receptor is followed by activation of PI3K, which phosphorylates Fyn kinase, activated Fyn kinase regulates both activation of zDHHC5 and phosphorylation of Flotillins. zDHHC5 via palmitoylation of flotillin1, 2 facilitates their binding to PM. Also, phosphorylated Flotillins induce homo or heterodimerization of flotillins and the formation of invagination vesicles. Besides, activated PI3K leads to actin polymerization and changes the dynamic of the actin cytoskeleton, thus the formation of endocytic vesicles