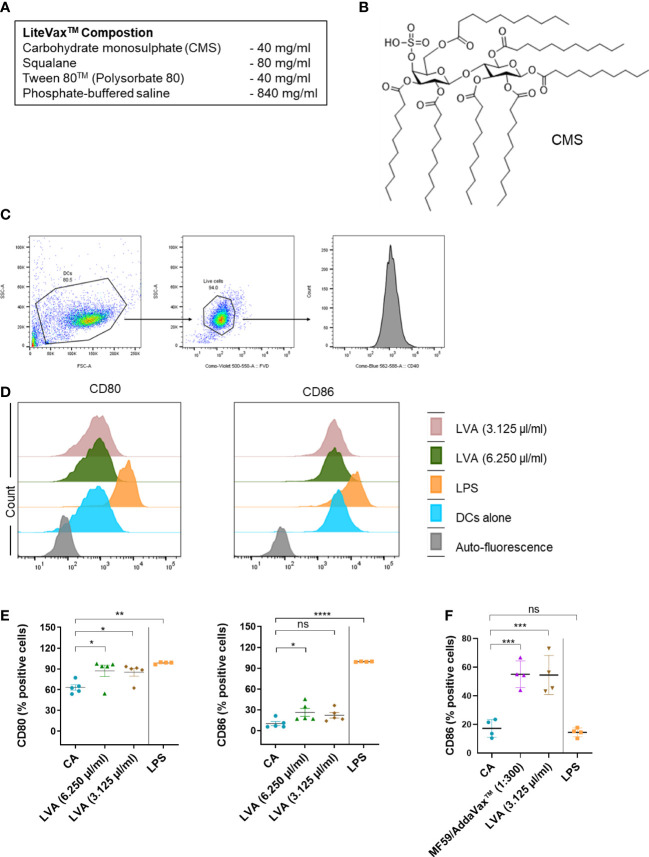
Figure 1.
Immunological mechanism of action of LiteVax™ (LVA) adjuvant on antigen presenting cells. (A) Composition of LVA. (B) Structure of active constituent of LVA, CMS: Maltose 4’–monosulphate 1,2,3,6,2’,3’,6’–heptadecanoic acid ester. (C–E) Effect of LVA on dendritic cell maturation. Monocytes were isolated from human peripheral blood mononuclear cells. Monocytes were cultured with granulocyte–macrophage colony–stimulating factor (GM–CSF, 1000 IU/106 cells) and IL–4 (500 IU/106 cells) in RPMI 1640 complete medium (10% fetal calf serum and 1% penicillin–streptomycin) for 5 days to obtain immature dendritic cells. Immature dendritic cells (0.5x106 cells/ml) were seeded in the 24–well plate and left untreated (CA) or treated with LPS (100 ng/ml, a positive control), LVA (3.125 µl/ml, which contains 125 µg of CMS, or 6.250 µl/ml, which contains 250 µg of CMS) for 48 h. Gating strategy is presented (C). Representative histograms (D) with scatter plots (E) of data presented as mean ± SEM (n=4–5) values of expression (% positive cells) of CD80 and CD86. (F) Effect of LVA on monocytes activation. Monocytes were cultured in RPMI complete medium without GM–CSF/IL–4 and were either not treated or treated with LPS (100 ng/ml), AddaVax™/MF59 (1:300 v/v, a squalene–based oil–in–water nano–emulsion as a control), LVA (3.125 µl/ml) for 48 h. After incubation, cells were subjected to phenotyping by flow cytometry and the expression of CD86 (% positive cells) was presented as mean ± SEM (n=4 donors). Statistical significance as determined by one–way ANOVA with Dunnett’s multiple comparisons post–test. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001, ns, not significant. CA, cells alone, LPS, lipopolysaccharide.