Abstract
Esophageal cancer (EC) is one of the most life-threatening malignancies worldwide. Esophageal squamous cell carcinoma (ESCC) is the dominant subtype, accounting for approximately 90% of new incident EC each year. Although multidisciplinary treatment strategies have advanced rapidly, patients with ESCC are often diagnosed at advanced stage and the long-term prognosis remains unsatisfactory. In recent decades, immunotherapy, such as immune checkpoint inhibitors (ICIs), tumor vaccines, and chimeric antigen receptor T-cell (CAR-T) therapy, has been successfully used in clinical practice as a novel therapy for treating tumors, bringing new hope to ESCC patients. However, only a small fraction of patients achieved clinical benefits due to primary or acquired resistance. Immune evasion plays a pivotal role in the initiation and progression of ESCC. Therefore, a thorough understanding of the mechanisms by which ESCC cells escape from anti-tumor immunity is necessary for a more effective multidisciplinary treatment strategy. It has been widely recognized that immune evasion is closely associated with the crosstalk between tumor cells and the tumor microenvironment (TME). TME is a dynamic complex and comprehensive system including not only cellular components but also non-cellular components, which influence hallmarks and fates of tumor cells from the outside. Novel immunotherapy targeting tumor-favorable TME represents a promising strategy to achieve better therapeutic responses for patients with ESCC. In this review, we provide an overview of immune evasion in ESCC, mainly focusing on the molecular mechanisms that underlie the role of TME in immune evasion of ESCC. In addition, we also discuss the challenges and opportunities of precision therapy for ESCC by targeting TME.
Keywords: esophageal squamous cell cancer, immune evasion, tumor microenvironment, immunosuppression, immunotherapy
1 Introduction
Esophageal cancer (EC) is an aggressive malignancy and accounts for the majority of cancer-related deaths worldwide, ranking seventh in incidence and sixth in mortality currently (1, 2). Generally, EC is divided into two subtypes based on pathological and biological features: esophageal squamous cell carcinoma (ESCC) and esophageal adenocarcinoma (EAC) (3). ESCC is the most common and the dominant subtype in Asians, accounting for approximately 90% of new incident EC each year, while EAC is more commonly diagnosed in western countries (4). Despite the great improvement in earlier diagnosis and multidisciplinary cancer treatment (including surgery, radiotherapy, chemotherapy and targeted therapy), the long-term prognosis of patients with ESCC remains unsatisfied (5), and the burden caused by ESCC continues to increase with population growth and aging (6). In recent decades, immunotherapy has gradually attracted attention due to the progressive insights into tumor immunology. Immunotherapy is a series of treatments aimed at revitalizing the anti-tumor immune system, restarting and sustaining the cancer-immune cycle, ultimately causing the death of tumor cells (7). Immune checkpoint inhibitors (ICIs), as a representative of immunotherapy, has been successfully used in the clinical practice for the treatment of ESCC with satisfactory outcomes (8, 9). However, only a small fraction of patients with ESCC response to immunotherapy due to primary or acquired resistance (10). Tumor cells can escape the immune system’s attack in a variety of ways such as inhibition of immune cells and restriction of antigen recognition, which is defined as immune evasion (11). Therefore, a thorough understanding of the mechanisms of immune evasion within the tumor microenvironment (TME) is necessary for a more effective multidisciplinary treatment strategy.
TME is a dynamic complex and comprehensive system, which includes not only cellular components such as tumor cells themselves and inflammatory/immune cells, but also non-cellular components such as extracellular matrix (ECM) and cytokines (12). During the past few decades, accumulative evidence has proven that TME plays a vital role in tumorigenesis, progression and immune escape of human cancer (13). To overcome the limitations of immunotherapy and develop more effective therapeutic strategies for ESCC, therapeutic strategies targeting tumor cells and tumor-favorable microenvironment emerged as a promising approach (14). In this review, we provide an overview of immune evasion in ESCC, mainly focusing on the molecular mechanisms that underlie the role of TME in immune evasion, especially in ESCC. In addition, we also discuss the challenges and opportunities of precision therapy for ESCC by targeting tumor-favorable TME.
2 Cellular components
2.1 ESCC cells
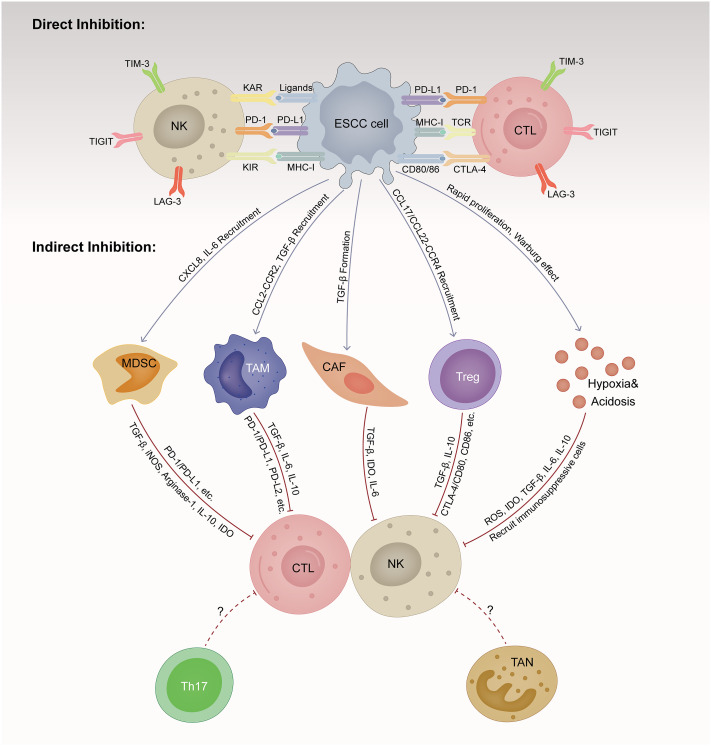
To effectively eradicate ESCC cells, repeated and magnified events of the cancer-immune cycle are indispensable, among which the initiation of identifying and processing tumor antigens recognition and ultimately the elimination of cancer cells taking place in the TME (15). However, ESCC cells are born to create an immunosuppressive milieu that suppresses the proliferation and function of cytolytic NK and T cells directly and indirectly (16). Here, we summarize the main mechanisms by which ESCC cells induce immune evasion ( Figure 1 ).
Figure 1.
Immunosuppressive tumor microenvironment (TME) in esophageal squamous cell cancer (ESCC). TME is a dynamic complex and comprehensive system, composed of various cellular components and non-cellular components. ESCC cells are born to create an immunosuppressive milieu suppressing the proliferation and function of cytolytic NK and T cells directly and indirectly. ESCC, esophageal squamous cell cancer; CTL, cytotoxic T lymphocyte; NK, natural killer cell; MDSC, myeloid-derived suppressor cell; TAM, tumor-associated macrophage; CAF, cancer-associated fibroblast; Treg, regulator T cell; TAN, tumor-associated neutrophils; Th17, T helper cell 17; MHC-1, major histocompatibility class I; TCR, T cell receptor; KAR, killer activation receptor; KIR, killer inhibitory receptor; PD-1, programmed cell death protein 1; PD-L1, programmed cell death ligand 1; PD-L2, programmed cell death ligand 2; CTLA-4, cytotoxic T lymphocyte-associated antigen 4; LAG-3, lymphocyte activation gene-3; TIM-3, T-cell immunoglobulin and mucin domain-containing protein-3; TIGIT, T-cell immunoglobulin and immunoreceptor tyrosine-based inhibitory motif domain; TGF-β, transforming growth factor-β; IL, interleukin; CXCL, CXC motif chemokine ligand; CCL, CC motif chemokine ligand; CCR, CC motif chemokine receptor; IDO, indoleamine 2,3-dioxygenase; iNOS, inducible nitric oxide synthase-2; ROS, reactive oxygen species.
Upregulation of immune checkpoint molecules is the main mechanism by which ESCC cells escape attacks from the immune system. Immune checkpoints (ICs) are immunosuppressive molecules that are normally expressed on the surface of multiple immune cells, which play an important role in preventing the occurrence of autoimmunity and long-lasting inflammation (17). However, ESCC cells can highly express the ligands for ICs to protect themselves from being killed by immune cells. At present, programmed cell death protein 1 (PD-1) and programmed cell death ligand 1 (PD-L1) are the most well-studied ICs in ESCC. Clinicians usually use the tumor proportion score (TPS) and combined positive score (CPS) to assess the expression of PD-L1, which is calculated by the following immunohistochemical scoring algorithm: TPS = (total number of PD-L1 stained tumor cells/total number of tumor cells) × 100%; CPS = (total number of PD-L1 stained cells/total number of tumor cells) × 100. It has been reported that patients with ESCC with TPS greater than 10% or CPS greater than 10 could benefit more from PD-1 inhibitors, which typically account for 30-50% of patients with ESCC (18). Abnormally highly expressed PD-L1 on tumor cells can bind to PD-1 expressed on various immune cells, protecting tumor cells from being lysis by CD8+ T cells or NK cells (19). Moreover, the binding of PD-1 and PD-L1 could also activate intercellular signaling pathways, inhibiting the function and proliferation of effector T cells (20). Tumor cells can even suppress T-cell immunity by secreting PD-L1 through exosomes (21). Antibody-based immunotherapy that blocks the interaction between PD-1 and PD-L1 has achieved favorable therapeutic effects for the treatment of ESCC (22). Cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) is another well-recognized IC, which is usually expressed on the surface of regulator T cells (Tregs) and T lymphocytes. CTLA-4 negatively regulate the anti-tumor immunity of T cells by binding to B7 ligand family. It has been widely demonstrated that the overexpression of CTLA-4 is associated with reduced interleukin-2 (IL-2) expression and T-cell cycle arrest, resulting in the reduction of T-cell function and immune evasion of cancer cells (23). A phase II clinical trial has shown the CTLA-4 inhibitor tremelimumab could provide favorable survival benefits for patients with gastric cancer and EAC (24). The clinical trial CheckMate-648 has proved that ipilimumab (anti-CTLA-4) combined with nivolumab (anti-PD-1) in first-line treatment could improve overall survival (OS) with durable objective responses and acceptable safety compared to chemotherapy alone for ESCC patients (25). However, due to the limited number of available clinical trials, the efficiency and safety of CTLA-4 inhibitors as an emerging immunotherapy for ESCC needs to be further investigated. Newly emerging ICs, including lymphocyte activation gene-3 (LAG-3), CD47, T-cell immunoglobulin and mucin domain-containing protein-3 (TIM-3), and T-cell immunoglobulin and immunoreceptor tyrosine-based inhibitory motif domain (TIGIT), have been identified to date and their efficacy is being investigated in various stages of preclinical and clinical trials. Previous studies have demonstrated their remarkable immune inhibitory effects on lymphocytes in ESCC and elevated expression of these molecules in ESCC patients was associated with worse survival outcomes, indicating that these ICs are promising targets for immunotherapy of ESCC (26–29). However, identification of a reliable immunotherapy that targets a certain IC remains a severe challenge because of the high heterogeneity of ICs expression on the cancer cell surface (30).
In addition to upregulating ICs, the expression of neoantigens and major histocompatibility class (MHC) I molecules on tumor cells is usually downregulated or completely absent, preventing the recognition of activated T cells and NK cells (11). Downregulation and depletion of neoantigens have been identified among various malignancies, such as lung cancer. For example, the role of neoantigen editing is diminished or the copy number of previous clonal neoantigens is lost during tumor evolution. Promoter hypermethylation of genes carrying neoantigens has been identified as a potential mechanism for immune editing in lung cancer (31). However, this mechanism has not been verified in ESCC, which could be further explored and elucidated in future studies. ESCC cells may also suppress the anti-tumor immune response and facilitate tumor progression by secreting some immunosuppressive cytokines that play a vital role in enhancing immune escape in the TME, including transforming growth factor-β (TGF-β), IL-6, and IL-10, etc. Previous studies have demonstrated that the expression levels of these immunosuppressive cytokines are significantly higher in ESCC tissues and are associated with a decrease in infiltrating lymphocytes in TME and a worse prognosis (32–35).
2.2 Cancer-associated fibroblasts
Cancer-associated fibroblasts (CAFs), as a main stromal component in the TME, have attracted increasing attention due to their important roles in tumorigenesis and tumor progression (36). CAFs, characterized by high expression of fibroblast activation protein-α and α-smooth muscle actin, are a group of activated fibroblasts with strong proliferation, migration, secretion and synthesis capabilities (37). Generally, resident fibroblasts around tumor cells are the main source of CAFs, and tumor cells can secrete various growth factors such as TGF-β to induce fibroblasts to differentiate into CAFs (38). Moreover, CAFs can interact with tumor-infiltrating immune cells within the TME through the secretion of various cytokines, growth factors, and other effector molecules, consequently creating an immunosuppressive and pro-survival TME that enables cancer cells to escape from the surveillance of the immune system (39). CAFs can reduce the expression of NK activation receptors and cytolytic proteins via the secretion of TGF-β, thereby inhibiting the activation and cytotoxic effects of NKs (40). In addition, TGF-β secreted by CAFs can also reduce the secretion of granzyme and perforin from cytotoxic T lymphocytes (CTLs) and inhibit cell survival proteins to promote the death of CTLs (41). Moreover, CAFs can recruit immunosuppressive cells or induce their differentiation. For example, CAFs can recruit tumor-associated neutrophils (TANs) by secreting chemokine including CXCL1, CXCL2, and CXCL5 (42); recruit monocytes to TME and differentiate them into M2 macrophages by releasing monocyte chemotactic protein 1 (MCP-1) (43); promote the differentiation of monocytes into myeloid-derived suppressor cells (MDSCs) by secreting IL-6 and signal transducer and activator of transcription 3 (STAT3) (44); stimulate CD4+ T cells to differentiate into Tregs and Th2-type helper T cells (45), etc. CAFs themselves can express different ligands of ICs molecules on their cell surface, such as PD-L1 and PD-L2, to induce T cell exhaustion and deactivation (46).
It has been proved that CAFs play an important role in shaping the immunosuppressive TME of ESCC through one or more of the above mechanisms. Qiu et al. found that CAFs could significantly upregulate the expression of PD-L1 in ESCC cells (47). Indirect co-culture of human bone marrow-derived mesenchymal stem cells (MSCs) with ESCC cells demonstrated that CAF-like cells promoted the cell growth and migration of ESCC cells and peripheral blood mononuclear cell-derived macrophage-like cells (33). CAF-like cells could also induce the M2 polarization of macrophage-like cells, which actively participate in the suppression of anti-tumor immune responses (33). Cui et al. found that indoleamine 2,3-dioxygenase (IDO), as an immunosuppressive factor that can induce apoptosis of effector T and NK cells while promoting Tregs activity, is expressed in CAFs and endothelial cells in the ESCC stroma, suggesting that ESCC cells may evade immune surveillance through IDO-expressing CAFs (48). Recent research in ESCC confirmed that CAFs could induce the generation of monocytic MDSCs via IL-6/exosomal miR-21-activated STAT3 signaling (49). Kato et al. analyzed the intratumoral and peritumoral tissues of 149 ESCC patients using immunohistochemical analysis, and found that CAFs were negatively and positively correlated with CD8+ tumor-infiltrating lymphocytes (TILs) and forkhead box protein 3 (FoxP3+) TILs in intratumoral tissues, respectively (50). Therefore, CAFs may create an immunosuppressive TME for ESCC cells by promoting the infiltration of FoxP3+ TILs (Tregs) while inhibiting that of the CD8+ TILs. Similarly, Huang et al. found a negative correlation between WNT2+ CAFs and active CD8+ T cells in primary ESCC, and anti-WNT2 monoclonal antibody (mAb) could restore anti-tumor immune responses and enhance the efficacy of anti-PD-1 in both mouse ESCC and colorectal cancer syngeneic tumor models (51). Additionally, previous studies have revealed that FGF2 overexpression in CAFs promotes sprout RTK signaling antagonist 1 (SPRY1) expression in CD8+ T cells and attenuates T cell receptor (TCR)-triggered T cell activation in vitro and in vivo, which could contribute to ESCC progression (52).
Therefore, novel immunotherapy targeting CAFs would immensely benefit patients with ESCC due to the central role CAFs paly in shaping the immunosuppressive TME. Recently, fibroblast activation protein (FAP), as a common biomarker of CAFs, has been extensively studied to develop immunotherapies targeting CAFs in TME in several human malignancies including ESCC (47, 53). A variety of materials, such as DNA vaccine, oncolytic adenovirus, and nanoparticles have been designed to target FAP+ CAFs and some of them have already entered clinical trials (54–56). Therefore, eliminating the tumor-promoting effects of CAFs by targeting FAP+ CAFs is a promising approach to potentially lead to better prognosis for ESCC patients. However, CAFs-targeted immunotherapy is still some way from entering clinical practice in ESCC.
2.3 Tumor-associated macrophages
Infiltration of tumor-associated macrophages (TAMs) is one of the hallmarks of cancer (57). The differentiation and maturation process of macrophages can be broadly divided into two different types based on different microenvironmental stimuli: the classical activation pathway produces M1 macrophages, and the bypass activation pathway produces M2 macrophages (58). M1 macrophages are believed to participate in anti-tumor immune responses, while M2 macrophages mainly play a pro-tumor role and constitute the predominant class of TAMs (59). M2-like TAMs are closely associated with the formation of immunosuppressive TME by expressing various surface markers and secreting cytokines, however, the underlying mechanisms remain largely obscure. M2-like TAMs can secrete growth factors, matrix metalloproteinases (MMPs), and other cytokines that are pro-tumor as well as inhibit T and NK cells, leading to an attenuated immune response (60). M2-like TAMs can recruit CD4+ Th2 type cells and Treg cells by secreting chemokines C‐C motif chemokine ligand 17 (CCL17) and CCL22 (61). In addition, M2 macrophages can express IC molecules such as PD-L1 to inhibit the proliferation and activation of T lymphocytes (62).
Previous studies have identified TAMs as important participants in the formation of the immunosuppressive TME of ESCC. Zheng et al. found that M2 macrophages was enriched in TME of ESCC, which could be associated with immune evasion and tumor progression (63). A study performed by Yagi et al. showed that increased TAMs density in EC tissues related to worse survival outcomes, and TAMs could also increase PD-L1 expression in tumor cells to induce the immune escape of them (64). The C‐C motif chemokine ligand 2 (CCL2)/C‐C motif chemokine receptor 2 (CCR2) signaling pathway is widely believed to play an important role in recruiting TAMs, subsequently contributing to tumor progression (65). Recent research in ESCC revealed that upregulated expression of CCL2 was associated with TAMs accumulation, and they were both good predictors for poor survival. Blocking the CCL2-CCR2 signaling pathway could significantly restore the anti-tumor efficacy of CD8+ T cells in TME by hindering TAMs recruitment. Moreover, M2 polarization of macrophages upregulated PD-L2 expression in TAMs, leading to immune evasion and tumor progression via PD-1 signaling pathway (66). The colony-stimulating factor 1 (CSF-1)/colony-stimulating factor 1 receptor (CSF-1R) signaling pathway has been proved to regulate the production, differentiation and activation of TAMs (67). Omstead et al. reported that the inhibition of CSF-1/CSF-1R signaling axis resulted in increased infiltration of CD8+ T cells with decreased M2 macrophage polarization in the TME of EAC rat model, and CSF-1R inhibitors could enhance the anti-tumor activity of PD-1/PD-L1 inhibitors by suppressing immune evasion (68). However, the roles and mechanisms of CSF-1/CSF-1R axis in immune escape of ESCC are still not fully elucidated, which is worth exploring in future studies. Therefore, TAMs represent a promisingly valuable therapeutic target for precise immunotherapy of ESCC due to their pivotal roles in cancer progression and immune evasion.
In recent years, TAMs have gradually become a hot target of tumor immunotherapy. With more and more in-depth understanding of the interaction between TAMs, TME and tumor cells, many anti-tumors therapy related explorations have been carried out in targeting TAMs (69). One strategy to target TAMs is to inhibit the recruitment of TAM precursors, that is, to prevent monocytes from being recruited into TME and prevent their activation into M2-like TAMs (69). There are currently several preclinical and clinical trials focused on inhibiting TAMs recruitment in TME by blocking CSF-1/CSF-1R signaling pathway and CCL2/CCR2 interaction in various human tumors (70–72). Nevertheless, immunotherapy targeting TAMs is still a certain distance from clinical practice. Moreover, immunotherapy targeting TAMs in TME has hardly been investigated in ESCC, and further studies in this field are expected to improve the prognosis of ESCC patients.
2.4 Tregs
Tregs are a distinct subpopulation of CD4+ T cells with immunosuppressive properties that are essential for maintaining immune homeostasis and self-tolerance, limiting excessive inflammation, and preventing autoimmunity (73). Tregs, which are universally characterized by CD4+CD25+Foxp3+CD127low/−, are an important component of the TME and play a vital role in the immune escape of tumors (74). Tregs exhibit their immunosuppressive function through various mechanisms. For example, Tregs can induce immunosuppression through the release of suppressive cytokines such as IL-10, TGF-β, and IL-35 (75–77). CTLA-4 expressed on the surface of Tregs is a ligand for CD80/CD86 with higher affinity than CD28, which downregulates CD80/CD86 co-stimulatory molecules on antigen-presenting cells (APCs), depriving T cells of co-stimulatory signals and thereby strongly inhibiting the maturation of APCs (78). In addition, Tregs mediate depletion and apoptosis of effector T cells by competitive binding of IL-2 and producing granzyme B, thereby reducing the number of effector T cells and inhibiting the immune responses (79).
Increased accumulation of Tregs has been verified both in the peripheral blood and esophageal mucosa of esophageal cancer patients, indicating that Tregs may play important roles in shaping the immunosuppressive TME of ESCC (63, 80). The chemokines CCL17 and CCL22, which are secreted by tumor cells and macrophages, have been reported as key factors in the recruitment of Tregs via the CCR4 receptor in ESCC (81). Previous studies have demonstrated that the increased infiltration of Tregs is associated with deep tumor invasion and worse prognosis for ESCC patients (82). Yue et al. found that IL-33 could promote the expression of CCL2 through the nuclear factor-κB (NF-κB) pathway, thereby recruiting Tregs to promote ESCC progression (83). Zhao et al. reported that elevated L1 cell adhesion molecule (L1CAM) expression in ESCC cells could facilitate CCL22 expression by activating the PI3K/Akt/NF-κB signaling pathway, thereby promoting Tregs recruitment to the tumor site (84). A recent study performed by Han et al. showed that IL-32 may have a contradictory role in the TME of ESCC, which enhanced the anti-tumor activity by promoting IFN-γ expression in CD8+ T cells, while suppressing the immune responses by inducing Foxp3 expression in CD4+ T cells (85). However, the specific mechanisms of Tregs in mediating immune evasion from ESCC are far from well-elucidated due to their heterogeneity and context-dependent functions, which is a current challenge for this field.
2.5 Myeloid-derived suppressor cells
MDSCs are defined as a heterogeneous group of immature myeloid cells associated with poor prognosis and survival in cancer patients. MDSCs are usually generated in the bone marrow, and they can migrate into peripheral lymphoid tissues and TME when cancer occurs, contributing to TAMs formation (86). MDSCs can suppress anti-tumor immune responses by various mechanisms. Activated MDSCs can directly inhibit NK and effector T cells via the production of Arginase-1 and inducible nitric oxide synthase-2 (iNOS) (87). MDSCs participate in the formation of immunosuppressive TME by secreting some immunosuppressive molecules, such as TGF-β, IL-10 and IDO (88). Moreover, MDSCs also have the ability to induce the expansion of Tregs with immunosuppressive properties (89).
Accumulated evidence has proved that MDSCs may serve as one of the most important immunosuppressive cells in TME of ESCC, which is associated with poor prognosis (90). Huang et al. found that increased circulating MDSCs were accompanied by high PD-L1 expression, indicating that MDSCs might exert immunosuppressive function via PD-1/PD-L1 pathway (91). It has been reported that IL-6 played crucial roles in MDSCs recruitment, and IL-6-stimulated MDSCs expressed significantly higher levels of reactive oxygen species (ROS) and arginase-1 (92). Karakasheva et al. found that MDSCs expressing high level of CD38 showed greater immunosuppressive capacity in vivo, and the CD38 expression was mediated by factors including IL-6, interferon-γ (IFN-γ), CXCL16, TNF-α, and IGFBP-3. Meanwhile, the number of CD38+ MDSCs in the peripheral blood of advanced stage EC patients was also increased (93). A study performed by Li et al. revealed that Maelstrom (MAEL) could promote the recruitment of polymorphonuclear myeloid-derived suppressor cells (PMN-MDSCs) to tumor sites via activation of Akt1/RelA/IL-8 signaling pathway, and TGF-β secreted by PMN-MDSCs could upregulate MAEL by inducing Smad2/Smad3 phosphorylation to further promote ESCC progression (94). Yue et al. recently reported that NEDD9 could maintain the stem-like phenotype of ESCC cells by recruiting MDSCs via CXCL8, suggesting NEDD9 as a novel therapeutic target for ESCC (95). Currently, the roles and mechanisms of MDSCs in the formation of immunosuppressive TME in ESCC remains obscure, and further investigation into MDSCs biology will provide direction for therapeutic approaches targeting MDSCs.
2.6 Tumor-associated neutrophils
Neutrophils are one of the most important inflammatory immune cells, and they are closely associated with the development of tumors (96). A large number of neutrophils are often gathered in the TME, which is defined as tumor-associated neutrophils (TANs). Similar to M1/M2 TAMs, TANs in the TME can be divided into two phenotypes: N1 and N2. Among them, N1 phenotype has the effect of inhibiting the progression of tumors by direct cytotoxicity, antibody-dependent cell-mediated cytotoxicity (ADCC) and activation of other immune cells. In contrast, N2 type TANs promote the proliferation and metastasis of tumor cells, inhibit anti-tumor immunity, and ultimately promote tumor progression (97). TGF-β within the TME can induce the polarization of TANs from N1 to N2 phenotype, thereby promoting tumor progression (98).
The important role of TANs in the development of ESCC is gradually being recognized. Recent studies revealed that higher neutrophil to lymphocyte ratio (NLR) was associated with deeper tumor invasion, lymph node metastasis, advanced TNM stage, and worse survival outcomes in ESCC patients undergoing esophagectomy (99, 100). Although the NLR is usually calculated from routine blood tests, it can still reflect changes of inflammation status in the TME (101). Neutrophil extracellular traps (NETs) is a meshwork of deoxyribonucleic acid, histones and antibacterial proteins released by activated neutrophils, which participate in the immune responses by trapping pathogens (102). However, in recent years, it has been found that NETs exist in the TME of a variety of tumors, and NETs can promote the spread and metastasis of tumor by trapping circulating tumor cells (103). Zhang et al. has demonstrated that elevated level of intra-tumoral NETs infiltration was associated with worse prognosis in both ESCC and EAC patients (104). Nevertheless, a prior study showed that IL-17 could induce accumulation and activation of myeloperoxidase+ TANs by stimulating ESCC cells to secret CXCL2 and CXCL3, and IL-17 could also strengthen the killing capacity of TANs by releasing various cytotoxic molecules, which predicted a favorable prognosis in ESCC patients (105). Therefore, the role of TANs in ESCC remains poorly elucidated and needs to be further investigated. Given that TANs play both tumor-suppressive and tumor-promoting roles in the process of tumor development, the full and rational utilization of their dual roles may provide new directions for ESCC immunotherapy strategy.
The role of TANs in tumor immunity has become a hot topic in recent years. How to make good use of the two sides of TANs will become a serious challenge in the future (106). Although there are various TANs-targeted therapies, little significant progress has been achieved. Up to now, several preclinical and clinical studies have evaluated the efficacy and safety of TANs-targeted therapies. Inhibition of neutrophil recruitment and polarization has been evaluated for tumor therapeutic efficacy (107–109). As for ESCC, the current challenge is to explore the role and specific mechanism of TANs in tumor immunity, so as to provide direction and reference for immunotherapy targeting TANs in TME.
2.7 Th17 cells
T helper cell 17 (Th17) is a newly identified subset of CD4+ helper T cells that secretes IL-17, which is important in autoimmune diseases and the body’s immune response (110). Although Th17 cells are prevalent in the TME, their role in tumor immunity is still controversial. Previous studies demonstrated that IL-17A expressed by Th17 cells induced ESCC cells to produce chemokines that can aggregate various immune cells such as effector T cells, B cells, DCs, and NK cells migrate to ESCC tissue and then exert their anti-tumor effects (111, 112). However, Chen et al. found that the proportion of Th17 cells increased within the peripheral blood and tumor tissues of ESCC patients, and the elevated levels of Th17 cells was positively correlated with lymph node metastasis and advanced stages (113). In addition, it has been reported that Th17 cells possess the ability to convert into Tregs, suggesting Th17 cells may participate in the immune evasion of ESCC (114). Up to now, the functions of Th17 cells in ESCC are still not well-defined, thereby understanding the role of Th17 cells in tumor immunity is crucial for the development of novel immunotherapy strategies for ESCC.
3 Non-cellular components
3.1 Cytokines
Cytokines are a heterogeneous group of small soluble peptides or glycoproteins with pleiotropic effects that promote the growth, differentiation and activation of normal cells (115). They have pro- or anti-inflammatory activity and can also produce immunosuppressive activity, depending mainly on the microenvironment surrounding the tumor. Different cells produce different cytokines in the local tumor environment, thereby regulating different biological functions of various cells (116). Previous studies have revealed that cytokines in the TME participate in the immune escape of ESCC.
TGF-β is a multifunctional cytokine that regulates cell proliferation, differentiation, adhesion, migration and apoptosis, which is one of the major players in regulating the composition and function of the TME (117). TGF-β is mainly derived from tumor cells and regulates the growth and metastasis of tumor cells in an autocrine or paracrine manner. In addition, tumor-infiltrating stromal cells, including fibroblasts, leukocytes, macrophages, bone marrow-derived endothelial cells, and mesenchymal cells, are another major source of this cytokine (118). In early-stage tumors, TGF-β exerts anti-tumor effects by promoting cell cycle arrest and apoptosis, while in advanced-stage tumors TGF-β promotes tumor metastasis and recurrence by promoting immunosuppression, promoting tumor angiogenesis, and inducing epithelial-mesenchymal transition (EMT) (119). TGF-β can directly activate Tregs to inhibit the cytotoxicity of CTLs and NK cell as well as the antigen-presenting function of APCs (120). And TGF-β can stimulate the transformation of fibroblasts into CAFs, thereby promoting the immune escape of tumor cells (121). Importantly, TGF-β can also induce macrophages to polarize toward M2 type and while activating Tregs, resulting in immunosuppressive responses (122). Blum et al. found that the TGF-β signaling axis was hyperactivated in EC patients, and the genes regulated by these pathways were also overexpressed (123). And Zhang et al. revealed that the expression of TGF-β in CAFs was significantly associated with the prognosis of ESCC patients treated with chemoradiotherapy (32). Moreover, it has been reported that CAFs could induce chemoresistance by a FOXO1/TGF-β1 signaling loop in ESCC, indicating that TGF-β may be involved in the interaction between ESCC cells and CAFs (32). Gholamin et al. found that the mRNA of TGF-β, IL-10, and VEGF were overexpressed in ESCC patients, and TGF-β was significantly co-expressed with IL-10 and vascular endothelial growth factor (VEGF), which all plays important roles in immune suppression (124). A prior study performed by Li et al. showed that esophageal cancer-derived microvesicles could induce regulatory B cells with the ability to suppress the proliferation of CD8+ T cells (125). Notably, previous research in ESCC demonstrated that MDSCs derived-TGF-β induced elevated PD-1 expression on CD8+ T cells, resulting in resistance to PD-1/PD-L1 blockade in TME. And simultaneous blockade of PD-1/PD-L1 and TGF-β signaling collaboratively restored the anti-tumor ability of CD8+ T cells in both vitro and vivo (126). Encouraging data from a phase I clinical study (NCT02517398) demonstrate the potential safety and clinical antitumor activity of M7824 (anti-PD-L1 and anti-TGF-β dual antibody) in multiple types of difficult-to-treat cancers, including advanced non-small-cell lung cancer (NSCLC), human papillomavirus (HPV)-associated cancers, biliary tract cancer (BTC) and gastric cancer (127). In addition, in preclinical studies, M7824 also showed stronger antitumor activity compared to anti-PD-L1 and anti-TGF-β monotherapies (128). However, Merck KGaA announced in 2021 that M7824 as the first-line treatment for NSCLC and BTC was unlikely to achieve the desired outcomes and therefore terminated three related clinical studies (NCT03631706, NCT03833661, and NCT04066491). The reason for the failure of these clinical trials may be that TGF-β is a clinically unproven target compared to PD-1/PD-L1 and CTLA-4, and the effectiveness of the clinical combination is not yet clear. Another PD-L1 and TGF-β dual antibody, SHR-1701, is in various stages of clinical trials for a variety of malignancies, and their results are promising and may provide a new direction for immunotherapy in ESCC.
Interleukin (IL) is a class of cytokine produced by lymphocytes, monocyte-macrophages and other non-mononuclear cells, which can act on a variety of cells. They play an important role in transmitting information, activating and regulating immune cells, mediating inflammatory response (129). Notably, the expression of different ILs in the TME is unbalanced. IL-4, IL-6 and IL-10 are abundantly expressed in TME and play more pro-tumor role, while IL-2, IL-12, IL-15 and IL-18, which are immunomodulatory or pro-inflammatory factors, are restricted in the TME and play more anti-tumor role (129). ILs plays a pivotal role in the progression and immune evasion of ESCC. IL-6 activates downstream molecules such as STAT3 by binding to its receptor, which allows tumor cells to survive in a highly toxic inflammatory environment (130). It has been proved that the expression of IL-6 and STAT3 in the tissues of patients with ESCC is increased and correlated, and IL-6 and STAT3 are both independent poor prognostic factors of ESCC (131). Additionally, increased levels of CAFs-derived IL-6 promoted the migration of ESCC cells, and IL-6 was associated with the formation of immunosuppressive TME (33). Moreover, Chen et al. found that silencing B7-H4 significantly reduced IL-6 secretion, inhibited STAT3 activation, and reduced the amount of p-STAT3 entering the nucleus from the cytoplasm, thereby inhibiting the proliferation of ESCC cells (132). Wang et al. revealed that the level of IL-10 in the serum of ESCC patients was significantly increased, and the IL-10 level was positively associated with Tregs density (35). In addition, a recent study has demonstrated that IL-32 enhanced the anti-tumor activity by promoting IFN-γ expression in CD8+ T cells, while suppressing the immune responses by inducing Foxp3+ Tregs cells, indicating its contradictory role in the TME of ESCC (85). Currently, immunotherapeutic drugs designed for ILs are mainly agonists, including IL-2, IL-12, IL-15, etc (133). However, there are still limitations in clinical application of these agents, mainly because of the poor specificity of cytokine action and the high risk of immune-related adverse events (irAE). To solve this problem, some manufacturers now couple the cytokine agonist with an antibody (such as PD-1 antibody), thus giving some specificity to this cytokine agonist. In conclusion, ILs are key elements in the coordination of TME and tumor-immune cell interactions, and interleukin-related immunotherapy for various cancer has led to a wealth of exciting new developments in preclinical and clinical studies that will yield more interesting results in the coming years.
Chemokines are a class of small cytokines or signaling proteins secreted by various cells that have the ability to induce directional chemotaxis of nearby responding cells (134). Based on the location of the first two cysteine (C) residues of their primary protein structure, chemokines are generally divided into four subclasses: C, CC, CXC and CX3C chemokines. Chemokines participate in the formation of immunosuppressive microenvironment by recruiting immunosuppressive cells such as Tregs, MDSCs and TAMs to the TME, indicating that they play an important role in the immune evasion of ESCC (135). As mentioned above, the chemokines CCL2, CCL17 and CCL22 have been reported as key factors in the recruitment of Tregs via their corresponding receptor in ESCC (81, 83, 84). In addition, Liu et al. has revealed that chemokine CCL20 was positively correlated with Treg markers in ESCC tissues, and CCL20 could recruit Tregs in vitro by binding to its receptor CCR6 (136). Recent research in ESCC revealed that upregulated expression of CCL2 could mediate TAMs accumulation via the CCL2-CCR2 signaling pathway, thereby inhibiting the anti-tumor ability of CTLs in TME and promoting immune evasion of ESCC cells (66). Moreover, Yue et al. demonstrated that CXCL8 mediated the recruitment of MDSCs induced by NEDD9 in vitro and in vivo, which promoted the stemness of ESCC cells (95). A previous study conducted by Chen et al. showed that the CCL17/22- CCR4 and CCL20-CCR6 axes might be of considerable significance in Th17 cells infiltration in TME of ESCC (137). Given the important roles chemokines play in the aggregation of immunosuppressive cells in TME, chemokines hold promise as potential therapeutic targets for ESCC patients. At present, chemokine-based immunotherapies include two main types: targeting pro-tumor chemokines and increasing the concentration of anti-tumor chemokines. Both can be used either as stand-alone therapies or in combination with other therapeutic strategies (138). Carlumab, a high-affinity monoclonal antibody that specifically targets CCL2, inhibits CCL2-mediated migration of TAMs to tumors. Despite showing robust therapeutic effects in preclinical mice models, treatment with carlumab failed to affect response rates in some clinical trials (NCT00537368 and NCT01204996) and was therefore discontinued (138, 139). HuMax-IL8 is an antibody approach specifically targeting CXCL8. Preclinical studies have shown that blocking CXCL8 with HuMax-IL8 reduces the recruitment of MDSCs to TME (140). Results from the phase I clinical trial demonstrated that serum CXCL8 levels were reduced, although no objective tumor response was detected (141). Together, immunotherapies that target chemokines have promising potential and feasibility, although their clinical potential remains to be demonstrated.
3.2 Hypoxia, acidosis, and metabolism
Hypoxia and low pH are characteristic manifestations in the TME, which are closely associated with a series of metabolic changes in tumors (142). For solid tumors, the rapid proliferation and energy metabolism of tumor cells in a relatively confined space with delayed vascular development and poor blood perfusion make hypoxia the norm in the TME (143). The hypoxic microenvironment can activate signaling pathways such as hypoxia-inducible factor 1 (HIF-1) in tumor cells to alter the biological behavior and material-energy metabolism to adapt to the hypoxic environment (144, 145). In turn, tumor cells can modify the TME by releasing biological molecules such as MMP, VEGF and metabolites such as lactic acid, changing the ECM, increasing angiogenesis and changing the pH value of the microenvironment, which have an impact on drug delivery, hemoperfusion and immunological status in the TME (146–148). To a certain extent, it can also enhance the tolerance of tumors to radiotherapy, chemotherapy and immunotherapy (149, 150). It has been proven that hypoxia can lead to the upregulation of some cytokines in the TME such as NF-κB, IL-6, IL-8, TGF-β, and IL-10 (151, 152). Hypoxic TME can promote the recruitment of immunosuppressive cells including Tregs, TAMs and MDSCs (153). Additionally, nutrient deficiencies and accumulation of metabolites in the TME pose a great challenge for immune cells to exert their anti-tumor effects, but cancer cells themselves are not affected by microenvironment selection (154). In recent years, the role of hypoxia in the immune escape of ESCC has gradually attracted attention. Previous research on ESCC has showed that HIF-1α expression was significantly associated with venous invasion, VEGF expression, and microvessel density, and ESCC patients with high HIF-1α-expressing tumors had worse survival outcomes (155). Lu et al. revealed that hsa-circ-0048117-rich exosomes generated by ESCC cells in a hypoxic TME could promote M2 macrophage polarization and favors the malignant behaviors of ESCC cells (156). A recent study has identified six hypoxia-related lncRNAs (HRlncRNAs) that were associated with the prognosis of digestive system pan-cancer, and the HRlncRNA high expression group was positively correlated with increased tumor-infiltrating immune cells such as CAFs, macrophages, and myeloid dendritic cell, while they were negatively correlated with CD4+ T cells, suggesting an immunosuppressive TME (157). However, the role of hypoxia in the formation of the immunosuppressive TME of ESCC and its specific mechanisms are still not well elucidated, which provides a direction for future research.
It is well recognized that hypoxic TME is usually accompanied by an increase in lactate concentration due to the “Warburg effect”. The Warburg effect means that even with sufficient oxygen, tumor cells obtain energy predominantly through the glycolysis pathway rather than the tricarboxylic acid (TCA) cycle, resulting in lactate accumulation in the TME. Lactate accumulation in TME can induce polarization of M2-type TAMs and N2-type TANs (158, 159). In addition, acidic TME induced by lactate produced by tumor cells can inhibit the anti-tumor immune responses of CTLs and NK cells (160, 161). It has been proved that the activity and recruitment of Tregs were increased in acidic TME, and the expression of Foxp3 in Tregs is also significantly increased, which enhanced the immunosuppressive effect of Tregs (73, 162). Additionally, excessive amounts of lactic acid in TME increased PD-1 expression and suppressive activity of Treg cells. PD-1 blockade could also enhance immunosuppressive activities of effector Treg cells in a high-lactate environment, and the efficacy of PD-1 blockade therapy was recovered by targeting lactic acid metabolism of Treg cells, indicating that lactic acid is a factor in immunotherapy resistance (163). Acidosis also plays an important role in the immune escape of EC. Carbonic Anhydrase IX (CAIX), as a target gene of HIF-1α, acts as a key molecule in maintaining the pH value of cells in a hypoxic microenvironment. Previous study has demonstrated that the expression of CAIX increased in EC, and CAIX could promote the migration and metastasis of EC cells while maintaining intracellular pH stability (164). Moreover, elevated level of CAIX expression was associated with greater aggressiveness and worse survival outcomes of ESCC (165). In hypoxic TME, a series of metabolites produced by tumor cells and inflammatory cells, including lactic acid, ROS, IDO, prostaglandin E2 (PGE2), soluble fatty acids and adenosine and other molecules, can accelerate the aging and exhaustion of immune cells, creating a zone of immunosuppression (166, 167). In recent years, the roles of hypoxia, acidosis and metabolism in the formation of immunosuppressive TME have gradually attracted attention, which is expected to provide a new target for the immunotherapy of ESCC.
4 ICIs drug resistance and TME
Over the past decade, ICIs has been successfully applied in the clinical practice as a novel therapy for treating ESCC, bringing new hope to ESCC patients. However, the overall response rate in patients with ESCC is lower than 30%, and the majority of patients initially treated with ICIs are likely to develop acquired resistance over time (120). The efficiency and durability of ICIs were greatly weakened by immunosuppression. Notably, the mechanisms of drug resistance is a complex multifactor process, including blockage of drug distribution, mutations of the drug target, increased drug efflux, and evasion of programmed cell death (168). Development of drug resistance is not only related to malignant hallmarks of tumor cells, but also closely associated with the immunosuppressive TME and the crosstalk between tumor cells and TME (150). It has been widely proved that TME plays an important role in the therapeutic response of various human cancer (169). However, the exact mechanism of ICIs resistance in ESCC remains elusive due to the heterogeneity within the immune TME and the highly diverse oncological characteristics in different ESCC individuals.
The presence of multiple ICs (including PD-1/PD-L1, CTLA-4, TIGIT, TIM, and LAG-3, etc.) is one reason for the development of drug resistance. Current immunotherapies generally block only one or two immune checkpoints, and unblocked immune checkpoints can still exert immunosuppressive effects. Some studies have found that many patients treated with chemotherapy plus immunotherapy develop primary or secondary drug resistance due to the existence of ICs such as TIGIT and TIM (170, 171). Several ongoing clinical trials (NCT04732494, NCT04140500, NCT03708328) combining anti-PD-1 and anti-TIGIT/TIM/LAG-3 treatment as novel immunotherapies are expected to provide better prognosis for ESCC patients. In addition to inhibitory molecules expression, ESCC cells could develop drug resistance by secreting immunosuppressive cytokines and growth factors (120). Previous studies have demonstrated that T cells activated by ICIs therapy preferentially recognize mutant antigens (172). The activation process of T cells is largely dependent on the MHC molecules of APCs, and tumor cell antigens presented through MHC class I molecules are regulated by a variety of genes. Thus, these neoantigens presented by APCs are also downregulated when genetic deletions, epigenetic alterations, or mutations, which may lead to acquired resistance of ICIs therapy. Beta-2-microglobulin (B2M) is a crucial gene involved in stabilizing the MHC class I molecules at the cell surface. Gettinger et al. found that B2M knockout cells were less sensitive to PD-1 blockade in lung cancer, indicating that B2M loss might confer drug resistance to ICIs therapy (173). Genetic mutations occur frequently during tumor growth, which generates neoantigens and affects the response to ICIs therapy (174). Patients with rich expression of neoantigens are more sensitive to ICIs treatment and obtain more clinical benefits, while loss of neoantigen mutations may lead to immune evasion and resistance to ICIs therapy (31, 175). Moreover, the above-mentioned immunosuppressive cells, including CAFs, TAMs, MDSCs, and Tregs may also participate in the development of drug resistance in ESCC patients through multiple pathways. Further exploration of the drug resistance mechanisms of immunotherapy is helpful for the development of new drugs, and provides ideas for further research and guidance for clinical treatment. Current studies have revealed some resistance mechanisms of immunotherapy in ESCC, but there are still more problems that need to be solved. With the continuous progress of molecular biology and gene sequencing technology, more drug resistance mechanisms will be discovered in the future.
5 Clinical trials on immunotherapy targeting the TME of ESCC
Tumor immunotherapy brings new hope to patients with cancers including ESCC, and in recent years immunotherapy has made breakthroughs in the field of basic research and clinical treatment. Several clinical trials have highlighted TME as a potential therapeutic target for ESCC, and some ongoing clinical trials are expected to provide broader prospects for the immunotherapy of ESCC. At present, immunotherapy strategies mainly focus on the combination of immunotherapy with chemotherapy or radiotherapy and dual immune blockade. We have summarized the available clinical trials on immunotherapy targeting the TME of ESCC registered with clinicaltrials.gov and presented them in Table 1 .
Table 1.
Overview of clinical trials with immunotherapies targeting immunosuppressive TME in ESCC.
| Identifier | Drug/Treatment | Target | Ethnicity | Phase | Status | Result | Ref. |
|---|---|---|---|---|---|---|---|
| NCT03189719 (KEYNOTE-590) | Pembrolizumab + CT vs. Placebo + CT (first-line) | PD-1 | Asian and Western | III | Active, not recruiting | Available | (8) |
| NCT03143153 (CheckMate-648) | Nivolumab + Ipilimumab vs. Nivolumab + CT vs. CT (first-line) | PD-1; CTLA-4 | Asian and Western | III | Active, not recruiting | Available | (176) |
| NCT03691090 (ESCORT-1st) | Camrelizumab + CT vs. Placebo + CT (first-line) | PD-1 | Asian | III | Completed | Available | (177) |
| NCT03829969 (JUPITER-06) | Toripalimab + CT vs. Placebo + CT (first-line) | PD-1 | Asian | III | Active, not recruiting | Available | (178) |
| NCT03748134 (ORIENT-15) | Sintilimab + CT vs. Placebo + CT (first-line) | PD-1 | Asian and Western | III | Recruiting | Available | (179) |
| NCT03469557 | Tislelizumab + CT (first-line) | PD-1 | Asian | II | Completed | Available | (180) |
| NCT02564263 (KEYNOTE-181) | Pembrolizumab vs. CT (second-line) | PD-1 | Asian and Western | III | Completed | Available | (9) |
| NCT02569242 (ATTRACTION-3) | Nivolumab vs. CT (second-line) | PD-1 | Asian and Western | III | Completed | Available | (181) |
| NCT03099382 (ESCORT) | Camrelizumab vs. CT (second-line) | PD-1 | Asian | III | Completed | Available | (182) |
| NCT03430843 (RATIONALE-302) | Tislelizumab vs. CT (second-line) | PD-1 | Asian and Western | III | Active, not recruiting | Available | (183) |
| NCT01938612 | Durvalumab monotherapy/Durvalumab + tremelimumab (≥ second-line) | PD-L1; CTLA-4 | Asian | I | Completed | Available | (184) |
| NCT02054806 (KEYNOTE-028) | Pembrolizumab (≥ third-line) | PD-1 | Asian and Western | Ib | Completed | Available | (185) |
| NCT02559687 (KEYNOTE-180) | Pembrolizumab (≥ third-line) | PD-1 | Asian and Western | II | Completed | Available | (186) |
| NCT03792347 (PALACE-1) | Preoperative pembrolizumab + CRT (neoadjuvant therapy) | PD-1 | Asian | Ib | Completed | Available | (187) |
| NCT02743494 (CheckMate-577) | Nivolumab vs. Placebo after surgery (adjuvant therapy) | PD-1 | Asian and Western | III | Active, not recruiting | Available | (188) |
| NCT04210115 (KEYNOTE-975) | Pembrolizumab + dCRT vs. placebo + dCRT | PD-1 | Asian and Western | III | Recruiting | Not available | N/A |
| NCT04435197 (PALACE-2) | Preoperative pembrolizumab + CRT | PD-1 | Asian | II | Recruiting | Not available | N/A |
| NCT04389177 (KEYSTONE-001) | Neoadjuvant pembrolizumab + nCT followed by surgery | PD-1 | Asian | II | Recruiting | Not available | N/A |
| NCT04807673 (KEYSTONE-002) | Neoadjuvant pembrolizumab + nCT + surgery vs. nCRT + surgery | PD-1 | Asian | III | Recruiting | Not available | N/A |
| NCT05302011 | Neoadjuvant pembrolizumab + nCT | PD-1 | Asian | II | Recruiting | Not available | N/A |
| NCT05130684 | Neoadjuvant nivolumab + nCRT | PD-1 | Asian | II | Recruiting | Not available | N/A |
| NCT03914443 (FRONTiER) | Neoadjuvant nivolumab + nCT | PD-1 | Asian | I | Active, not recruiting | Not available | N/A |
| NCT05213312 | Neoadjuvant nivolumab + nCT vs. nCT | PD-1 | Asian | II/III | Recruiting | Not available | N/A |
| NCT03416244 (RAMONA) | Nivolumab + Ipilimumab vs. Nivolumab | PD-1; CTLA-4 | Western | II | Completed | Not available | N/A |
| NCT04426955 | Camrelizumab + dCRT vs. Placebo + dCRT | PD-1 | Asian | III | Active, not recruiting | Not available | N/A |
| NCT05050760 | Camrelizumab + CT | PD-1 | Asian | N/A | Recruiting | Not available | N/A |
| NCT04741490 | Postoperative camrelizumab + RT | PD-1 | Asian | N/A | Recruiting | Not available | N/A |
| NCT04286958 | Camrelizumab after dCRT | PD-1 | Asian | II | Recruiting | Not available | N/A |
| NCT04767295; NCT05476380 | Neoadjuvant camrelizumab + nCT | PD-1 | Asian | II | Recruiting | Not available | N/A |
| NCT04506138 | Neoadjuvant camrelizumab + nCT | PD-1 | Asian | I/II | Active, not recruiting | Not available | N/A |
| NCT04225364 | Neoadjuvant camrelizumab + nCT | PD-1 | Asian | II | Completed | Not available | N/A |
| NCT05176002 | Neoadjuvant camrelizumab + nRT | PD-1 | Asian | II | Recruiting | Not available | N/A |
| NCT05507411 (WATCHER) | Neoadjuvant camrelizumab + cCRT | PD-1 | Asian | II | Recruiting | Not available | N/A |
| NCT04005170 | Toripalimab + dCRT | PD-1 | Asian | II | Completed | Not available | N/A |
| NCT04848753 | Neoadjuvant toripalimab + nCT vs. Placebo + nCT | PD-1 | Asian | III | Recruiting | Not available | N/A |
| NCT04280822 | Neoadjuvant toripalimab + nCT vs. nCT | PD-1 | Asian | III | Recruiting | Not available | N/A |
| NCT04644250; NCT05424432 | Neoadjuvant toripalimab + nCRT | PD-1 | Asian | II | Recruiting | Not available | N/A |
| NCT04844385 | Neoadjuvant toripalimab + nCT + cCRT | PD-1 | Asian | II | Recruiting | Not available | N/A |
| NCT04177797 | Neoadjuvant toripalimab + nCT | PD-1 | Asian | II | Active, not recruiting | Not available | N/A |
| NCT04804696 | Neoadjuvant toripalimab + nCT | PD-1 | Asian | II | Recruiting | Not available | N/A |
| NCT03783442 | Tislelizumab + CT | PD-1 | Asian | III | Active, not recruiting | Not available | N/A |
| NCT03957590 (RATIONALE-311) | Tislelizumab + dCRT vs. Placebo + dCRT | PD-1 | Asian | III | Active, not recruiting | Not available | N/A |
| NCT05394415 (LATE) | Tislelizumab + CRT for conversion therapy | PD-1 | Asian | I/II | Recruiting | Not available | N/A |
| NCT05520619 (EC-CRT-002) | Tislelizumab + dCRT with maintenance vs. Tislelizumab + dCRT without maintenance | PD-1 | Asian | II | Recruiting | Not available | N/A |
| NCT04973306 (iCROSS) | Neoadjuvant tislelizumab +nCRT vs. nCRT | PD-1 | Asian | II/III | Recruiting | Not available | N/A |
| NCT05323890; NCT04776590 | Neoadjuvant tislelizumab + nCRT | PD-1 | Asian | II | Recruiting | Not available | N/A |
| NCT04732494 (AdvanTIG-203) | Tislelizumab + Ociperlimab vs. Tislelizumab + Placebo | PD-1; TIGIT | Asian and Western | II | Recruiting | Not available | N/A |
| NCT05495152 | Postoperative sintilimab vs. postoperative follow-up | PD-1 | Asian | III | Active, not recruiting | Not available | N/A |
| NCT04212598 | Sintilimab after dCRT | PD-1 | Asian | II | Recruiting | Not available | N/A |
| NCT03985046 | Sintilimab + CT after dCRT | PD-1 | Asian | II | Active, not recruiting | Not available | N/A |
| NCT05174325; NCT04548440 | Neoadjuvant sintilimab + nCT | PD-1 | Asian | II | Recruiting | Not available | N/A |
| NCT03946969 | Neoadjuvant sintilimab + nCT | PD-1 | Asian | II | Active, not recruiting | Not available | N/A |
| NCT05357846 | Neoadjuvant sintilimab + nCRT vs. nCRT | PD-1 | Asian | III | Not yet recruiting | Not available | N/A |
| NCT04543617 (SKYSCRAPER-07) | Atezolizumab + tiragolumab vs. Atezolizumab + tiragolumab placebo vs. Atezolizumab placebo + tiragolumab placebo | PD-L1; TIGIT | Asian and Western | III | Recruiting | Not available | N/A |
| NCT04540211 (SKYSCRAPER-08) | Atezolizumab + Tiragolumab + CT vs. Placebo + CT | PD-L1; TIGIT | Asian | III | Active, not recruiting | Not available | N/A |
| NCT04550260 (KUNLUN) | Durvalumab + dCRT vs. Placebo + dCRT | PD-L1 | Asian and Western | III | Recruiting | Not available | N/A |
| NCT02520453 | Durvalumab after surgery vs. Placebo after surgery | PD-L1 | Asian | II | Active, not recruiting | Not available | N/A |
| NCT04851132 | Durvalumab + RT | PD-L1 | Asian | N/A | Recruiting | Not available | N/A |
| NCT04568200 | Neoadjuvant durvalumab + nCRT vs. Placebo + nCRT | PD-L1 | Asian | II | Recruiting | Not available | N/A |
| NCT03377400 | Durvalumab + tremelimumab + dCRT | PD-L1; CTLA-4 | Asian | II | Active, not recruiting | Not available | N/A |
| NCT02658214 | Durvalumab + tremelimumab + CT | PD-L1; CTLA-4 | Asian | I | Completed | Not available | N/A |
| NCT03212469 (ABIMMUNE) | Durvalumab + tremelimumab + SBRT | PD-L1; CTLA-4 | Western | I/II | Recruiting | Not available | N/A |
| NCT04140500 | RO7247669 | PD-1; LAG-3 | Asian and Western | I | Recruiting | Not available | N/A |
| NCT03708328 | RO7121661 | PD-1; TIM-3 | Asian and Western | I | Active, not recruiting | Not available | N/A |
| NCT04785820 | RO7247669/RO7121661 vs. Nivolumab | PD-1; LAG-3; TIM-3 | Asian and Western | II | Recruiting | Not available | N/A |
CT, chemotherapy; nCT, neoadjuvant chemotherapy; RT, radiotherapy; nRT, neoadjuvant radiotherapy; CRT, chemoradiotherapy; nCRT, neoadjuvant chemoradiotherapy; dCRT, definitive chemoradiotherapy; cCRT, concurrent chemoradiotherapy; SBRT, stereotactic body radiation therapy; PD-1, programmed cell death protein 1; PD-L1, programmed cell death ligand 1; CTLA-4, cytotoxic T lymphocyte-associated antigen 4; TIGIT, T-cell immunoglobulin and immunoreceptor tyrosine-based inhibitory motif domain; LAG-3, lymphocyte activation gene-3; TIM-3, T-cell immunoglobulin and mucin domain-containing protein-3; Ref., reference.
Currently, immunotherapy for ESCC is still focused on ICIs. Over the past decade, numerous clinical studies have shown that ICIs can increase remission rates and improve prognosis of ESCC patients. PD-1/PD-L1 and CTLA-4 represent the two major targetable ICs. In the field of tumor immunotherapy, PD-1/PD-L1 have attracted much attention and have been approved by the Food and Drug Administration (FDA) for the treatment of a variety of cancers. The common PD-1 inhibitors currently in clinical trials include pembrolizumab, nivolumab, camrelizumab, toripalimab, tislelizumab, and sintilimab, while atezolizumab and durvalumab are major PD-L1 inhibitors. Clinical studies have shown that some PD-1/PD-L1 inhibitors can be applied to the first-line treatment of advanced ESCC. The KEYNOTE-590 study showed that pembrolizumab in combination with chemotherapy (5-fluorouracil + cisplatin) demonstrated better efficacy and safety than placebo plus chemotherapy as first-line treatment for patients with locally advanced/unresectable or metastatic ESCC [OS: median 12.4 vs 9.8 months, P < 0.0001; progression-free survival (PFS): median 6.3 vs 5.8 months, P < 0.0001] (8). The CheckMate-648 is a study of nivolumab in combination with chemotherapy or ipilimumab (anti-CTLA-4 monoclonal antibody) in the first-line treatment of ESSC, and the resulted demonstrated that the nivolumab plus chemotherapy (5-fluorouracil + cisplatin) group (median 13.2 vs 10.7 months, P = 0.002) and nivolumab plus ipilimumab group (median 12.7 vs. 10.7 months, P = 0.01) both achieved significantly prolonged OS compared with chemotherapy alone (176). The phase III clinical trial ESCORT-1st revealed that camrelizumab in combination with chemotherapy (paclitaxel + cisplatin) in first-line treatment of patients with advanced or metastatic ESCC significantly prolonged PFS and OS (PFS: median 6.9 vs 5.6 months, P < 0.001; OS: median 15.3 vs 12.0 months, P = 0.001) with an overall good safety profile (177). In addition, the JUPITER-06 study was designed to compare the efficacy and safety of toripalimab in combination with chemotherapy (paclitaxel + cisplatin) versus placebo in combination with chemotherapy in the first-line treatment of advanced or metastatic ESCC, and the results showed that OS and PFS were improved in the toripalimab group (OS: median 17 versus 11 months, P = 0.0004; PFS: median 5.7 vs 5.5 months, P < 0.0001) with a worse but manageable safety profile (178). The ORIENT-15 study is a phase III clinical trial investigating sintilimab in combination with chemotherapy (cisplatin + paclitaxel/cisplatin + 5-fluorouracil) for the first-line treatment of advanced or metastatic ESCC (179). The results showed that sintilimab plus chemotherapy was more effective than chemotherapy alone (OS: median 16.7 versus 12.5 months, P < 0.001; PFS: median 7.2 vs 5.7 months, P < 0.001), and the treatment effect was more significant in PD-L1-positive population. Moreover, the phase II study (NCT03469557) showed that first-line tislelizumab plus chemotherapy (cisplatin + fluorouracil) demonstrated durable responses with manageable tolerability in patients with locally advanced or metastatic ESCC [PFS: 10.4 months (95% CI, 5.55–15.11); duration of response (DoR): 12.8 months (95% CI, 3.5-12.8)] (180).
Clinical trials exploring the use of PD-1/PD-L1 inhibitors for second-line and beyond treatment of ESCC have also made some progress. The KEYNOTE-181 trial is the first study to use immunotherapy in second-line ESCC treatment, and the results showed that pembrolizumab prolonged OS versus chemotherapy (paclitaxel/docetaxel/irinotecan) as second-line therapy for advanced ESCC in PD-L1-positive patients (median 8.2 vs 7.1 months, P = 0.0095), with fewer treatment-related adverse events (9). The phase III clinical trial ATTRACTION-3 revealed that nivolumab was associated with a significant improvement in OS with a favorable safety profile compared with chemotherapy (paclitaxel/docetaxel) as a second-line treatment option for ESCC patients (median 10.9 vs 8.4 months, P = 0.019) (181). Moreover, the ESCORT study revealed that camrelizumab monotherapy could significantly improve PFS and OS compared with chemotherapy (docetaxel/irinotecan) in patients with advanced or metastatic ESCC who failed first-line chemotherapy with a manageable safety profile (OS: median 8.3 versus 6.2 months, P = 0.001; PFS: median 1.9 vs 1.9 months, P < 0.001) (182). The RATIONALE-302 is a phase III clinical trial comparing the efficacy of tislelizumab with chemotherapy (paclitaxel/docetaxel/irinotecan) in the second-line treatment of patients with advanced or metastatic ESCC, and the resulted demonstrated that tislelizumab significantly improved OS compared with chemotherapy (median 8.6 vs 6.3 months, P = 0.0001) with a manageable safety profile (183). The NCT01938612 is a phase I clinical trial to evaluate the safety, tolerability, and antitumor activity of durvalumab monotherapy, and durvalumab plus tremelimumab (anti-CTLA-4 monoclonal antibody) combination therapy in patients from Asia with ESCC as second-line and beyond treatment, which showed that both treatments displayed acceptable safety profiles clinical benefit (184). The results of phase Ib clinical trial KEYNOTE-028 showed that pembrolizumab demonstrated durable antitumor activity and manageable toxicity in patients with heavily pretreated, PD-L1-positive advanced ESCC [objective response rate (ORR): 30% (95% CI, 13%-53%); DoR: 15 months (range, 6-26 months); OS: 7.0 months (95% CI, 4.3-17.7 months); PFS: 1.8 months (95% CI, 1.7-2.9 months)] (185). The KEYNOTE-180 study is a phase II clinical trial that investigated and evaluated the efficacy and safety of pembrolizumab in third-line and post-third-line treatment of advanced ESCC, which revealed that pembrolizumab could provide durable anti-tumor activity with tolerable safety in patients with heavily pretreated ESCC [ORR: 14.3% (95% CI, 6.7%-25.4%); OS: 6.8 months (95% CI, 5.4-8.9 months); PFS: 2.1 months (95% CI, 2.0 - 2.4 months)] (186). Additionally, The ATTRACTION-1 study, an open, multicenter phase II study of nivolumab given to patients with advanced ESCC who had failed or were intolerant to previous treatments, confirmed the good antitumor activity and controlled safety profile of nivolumab in third-line and beyond for the treatment of advanced ESCC (ORR: 17.2%; DoR: 11.2 months; OS: median 10.8 months; PFS: median 1.5 months) (189).
Clinical trials of ICIs as neoadjuvant immunotherapy have also made some progress. The PALACE-1 is a phase Ib clinical trial aiming to investigate the safety and activity of preoperative pembrolizumab combined with chemoradiotherapy for resectable ESCC, and the results showed that it was safe, did not delay surgery, and induced a pathologic complete response (pCR) in 55.6% of resected tumors (187). However, because PALACE-1 is only a Phase Ib clinical study, the role of preoperative neoadjuvant immunotherapy in the multidisciplinary management of ESCC is still far from well elucidated. Furthermore, results from ongoing clinical trials that incorporate immunotherapy into neoadjuvant regimens in ESCC (NCT04435197, NCT04389177, NCT04807673, NCT04848753, NCT04973306, and NCT05357846 etc.) are eagerly awaited to clarify safety and efficacy and whether immunotherapy can improve response to neoadjuvant treatment, which is highly relevant for overall survival of patients with ESCC. In addition, PD-1 inhibitors also showed promise in post-operative adjuvant therapy after radical surgery. The CheckMate-577 study revealed that nivolumab could be used as postoperative maintenance therapy for EC patients treated with neoadjuvant concurrent chemoradiotherapy and R0 resection [disease-free survival (DFS): 22.4 vs 11.0 months, P < 0.001], with significant improvement in prognosis (188). However, most of the participants in this study were patients from Western countries, and an ongoing clinical trial (NCT02520453) in China to explore the role of immunotherapy in the postoperative adjuvant treatment of ESCC is expected to contribute to this field.
CTLA-4 is another well-recognized immune checkpoint, and several clinical trials has proven the effectiveness of anti-CTLA-4 monoclonal antibody as a novel immunotherapy for comprehensive treatment of ESCC. The common CTLA-4 inhibitors currently in clinical trials mainly include ipilimumab and tremelimumab, and CTLA-4 inhibitors often combined with PD-1/PD-L1 inhibitors as dual immune blockade. As mentioned above, two clinical trials, CheckMate-648 and NCT01938612, have confirmed the efficacy and safety of combined CTLA-4 and PD-1 inhibitors as the immunotherapy of ESCC (176, 184). Several ongoing clinical trials (NCT03416244, NCT03377400, NCT02658214 and NCT03212469) are eagerly awaited to further clarify safety and efficacy of CTLA-4 inhibitors in immunotherapy of ESCC.
Some newly emerging ICs, including LAG-3, TIM-3, and TIGIT, have been identified to date and their efficacy are being investigated in several ongoing clinical trials. The AdvanTIG-203 (NCT04732494), SKYSCRAPER-07 (NCT04543617), and SKYSCRAPER-08 (NCT04540211) are clinical studies to investigate the efficacy and safety of anti-TIGIT and anti-PD-1/PD-L1 combination therapy for patients with ESCC. The NCT04140500 and NCT04785820 studies aimed to explore the efficacy and safety of anti-LAG-3 and anti-PD-1 combination therapy, while the NCT03708328 and NCT04785820 are designed to investigate the efficacy and safety of anti-TIM-3 and anti-PD-1 combination therapy for patients with ESCC. These ongoing clinical trials hold the promise of more diverse immunotherapy approaches for the comprehensive treatment of ESCC.
Although significant progress has been made in ESCC immunotherapy, only a small fraction of patients can actually achieve long-term benefits. Therefore, accurate screening of the best benefit groups by potential markers is an urgent problem to be solved, which is more in line with the concept of precision therapy. PD-L1 is a common immune biomarker, and detection of PD-L1 expression level is the most widely used method in clinical research. KEYNOTE-181 study confirmed that CPS ≥ 10 can be used as a biomarker to predict the efficacy of immunotherapy for esophageal cancer (9). Microsatellite instability-high (MSI-H), mismatch repair deficiency (dMMR) and tumor mutation burden (TMB) may also be potential biomarkers for predicting the efficacy of immunotherapy in ESCC (190). Additionally, with the advance of immunotherapy to the first-line treatment of advanced ESCC, the problem of drug resistance has gradually emerged. Some studies have found that in the chemotherapy combined with immunotherapy, due to the existence of ICs such as TIGIT and TIM, many patients develop primary or secondary drug resistance (170, 171). One potential strategy to overcome the limitations of PD-1 immunotherapy is to target additional immune checkpoints associated with the immunosuppressive TME. Several ongoing clinical trials combining anti-PD-1 and anti-TIGIT/TIM/LAG-3 treatment as novel immunotherapies are expected to provide better prognosis for ESCC patients. Moreover, the immunotherapy of ESCC is mainly based on ICIs currently. With the further research on the pathogenesis of ESCC, novel immunotherapies targeting immunosuppressive TME (such as CAFs, TAMs, Tregs, MDSCs and cytokines) will be available in the near future, which is promising to create a new era for the immunotherapy of ESCC.
6 Conclusions and perspectives
The TME is an indispensable participant in immune evasion of ESCC. TME is a dynamic and ever-changing biological system consisting of various cellular and non-cellular components, which is responsible for immune escape to some extent. Increasing evidence has demonstrated that the formation of immunosuppressive microenvironment in ESCC is a complex process, involving not only the tumor cells themselves, but also CAFs, TAMs, Tregs, MDSCs, TANs, cytokines, hypoxia, acidosis and metabolism. At present, immunotherapies have been developed that are often used in combination with conventional chemotherapy aim to promote treatment efficacy. Although significant improvement has been reached, responses to immunotherapies and prognosis in ESCC patients are still far from satisfactory.
The current challenges are to devote considerable effort to the immunological and biological exploration of ESCC to more accurately tune the various existing or emerging immunotherapies. Although previous studies have provided clues to the important role of various immunosuppressive cells in the TME of ESCC, further studies are needed to investigate their functions in the occurrence and progression of ESCC and the formation of the immunosuppressive TME. Meanwhile, the inadequacy and imbalance of previous studies may lead to incomplete assessment of the complex immune contexture in ESCC. For example, the role and mechanism of TANs and Th17 cells in the formation of ESCC immunosuppressive TME are still controversial, which are supposed to be further clarified in future studies. Another urgent challenge is to find biomarkers that can accurately predict patients’ responsiveness and drug resistance to immunotherapy. At present, there are few biomarkers existing or applied in daily clinical practice in ESCC, and further research is urgently needed.
With the further exploration of the formation mechanism of ESCC immunosuppressive TME, there will be more immunotherapeutic methods targeting immunosuppressive TME. By targeting key molecules and immune cells in the TME, different combinations of chemotherapy, molecular targeted therapy and immunotherapy are promising to further improve the prognosis of ESCC patients. A number of ongoing preclinical and clinical studies hold great promise to maximize ESCC patients’ benefits from an immunotherapeutic approach targeting tumor-favorable TME in the foreseeable future.
Author contributions
Conception and design: ZS and RL. Administrative support: ZS and HT. Provision of study materials or patients: RL and BH. Collection and assembly of data: RL. Data analysis and interpretation: RL. All authors contributed to the article and approved the submitted version.
Funding Statement
This work was funded by the National Key R&D Program of China (2021YFC2500900, 2021YFC2500904 and 2021YFC2500905), National Natural Science Foundation of China (81802397), China Postdoctoral Science Foundation (2020M672073), and Shandong Provincial Natural Science Foundation (ZR2017BH035).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer J Clin (2021) 71(3):209–49. doi: 10.3322/caac.21660 [DOI] [PubMed] [Google Scholar]
- 2. Xia C, Dong X, Li H, Cao M, Sun D, He S, et al. Cancer statistics in China and united states, 2022: profiles, trends, and determinants. Chin Med J (2022) 135(5):584–90. doi: 10.1097/CM9.0000000000002108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lagergren J, Lagergren P. Oesophageal cancer. BMJ (Clinical Res ed) (2010) 341:c6280. doi: 10.1136/bmj.c6280 [DOI] [PubMed] [Google Scholar]
- 4. Domper Arnal MJ, Ferrández Arenas Á, Lanas Arbeloa Á. Esophageal cancer: Risk factors, screening and endoscopic treatment in Western and Eastern countries. World J gastroenterology. (2015) 21(26):7933–43. doi: 10.3748/wjg.v21.i26.7933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lagergren J, Smyth E, Cunningham D, Lagergren P. Oesophageal cancer. Lancet (London England). (2017) 390(10110):2383–96. doi: 10.1016/S0140-6736(17)31462-9 [DOI] [PubMed] [Google Scholar]
- 6. Morgan E, Soerjomataram I, Rumgay H, Coleman HG, Thrift AP, Vignat J, et al. The global landscape of esophageal squamous cell carcinoma and esophageal adenocarcinoma incidence and mortality in 2020 and projections to 2040: New estimates from GLOBOCAN 2020. Gastroenterology° (2022) 163(3):649–58.e2. doi: 10.1053/j.gastro.2022.05.054 [DOI] [PubMed] [Google Scholar]
- 7. Yang Y. Cancer immunotherapy: harnessing the immune system to battle cancer. J Clin Invest (2015) 125(9):3335–7. doi: 10.1172/JCI83871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sun JM, Shen L, Shah MA, Enzinger P, Adenis A, Doi T, et al. Pembrolizumab plus chemotherapy versus chemotherapy alone for first-line treatment of advanced oesophageal cancer (KEYNOTE-590): a randomised, placebo-controlled, phase 3 study. Lancet (London England). (2021) 398(10302):759–71. doi: 10.1016/S0140-6736(21)01234-4 [DOI] [PubMed] [Google Scholar]
- 9. Kojima T, Shah MA, Muro K, Francois E, Adenis A, Hsu CH, et al. Randomized phase III KEYNOTE-181 study of pembrolizumab versus chemotherapy in advanced esophageal cancer. J Clin Oncol Off J Am Soc Clin Oncol (2020) 38(35):4138–48. doi: 10.1200/JCO.20.01888 [DOI] [PubMed] [Google Scholar]
- 10. Zhou X, Ren T, Zan H, Hua C, Guo X. Novel immune checkpoints in esophageal cancer: From biomarkers to therapeutic targets. Front Immunol (2022) 13:864202. doi: 10.3389/fimmu.2022.864202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kim SK, Cho SW. The evasion mechanisms of cancer immunity and drug intervention in the tumor microenvironment. Front Pharmacol (2022) 13:868695. doi: 10.3389/fphar.2022.868695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hui L, Chen Y. Tumor microenvironment: Sanctuary of the devil. Cancer letters. (2015) 368(1):7–13. doi: 10.1016/j.canlet.2015.07.039 [DOI] [PubMed] [Google Scholar]
- 13. Hinshaw DC, Shevde LA. The tumor microenvironment innately modulates cancer progression. Cancer Res (2019) 79(18):4557–66. doi: 10.1158/0008-5472.CAN-18-3962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Xiao Y, Yu D. Tumor microenvironment as a therapeutic target in cancer. Pharmacol Ther (2021) 221:107753. doi: 10.1016/j.pharmthera.2020.107753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Preusser M, Berghoff AS, Thallinger C, Zielinski CC. Cancer immune cycle: a video introduction to the interaction between cancer and the immune system. ESMO Open (2016) 1(3):e000056. doi: 10.1136/esmoopen-2016-000056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tang S, Ning Q, Yang L, Mo Z, Tang S. Mechanisms of immune escape in the cancer immune cycle. Int immunopharmacology. (2020) 86:106700. doi: 10.1016/j.intimp.2020.106700 [DOI] [PubMed] [Google Scholar]
- 17. Dhupar R, van der Kraak L, Pennathur A, Schuchert MJ, Nason KS, Luketich JD, et al. Targeting immune checkpoints in esophageal cancer: A high mutational load tumor. Ann Thorac surgery. (2017) 103(4):1340–9. doi: 10.1016/j.athoracsur.2016.12.011 [DOI] [PubMed] [Google Scholar]
- 18. Li ZC, Sun YT, Lai MY, Zhou YX, Qiu MZ. Efficacy and safety of PD-1 inhibitors combined with chemotherapy as first-line therapy for advanced esophageal cancer: A systematic review and network meta-analysis. Int immunopharmacology. (2022) 109:108790. doi: 10.1016/j.intimp.2022.108790 [DOI] [PubMed] [Google Scholar]
- 19. Zou W, Wolchok JD, Chen L. PD-L1 (B7-H1) and PD-1 pathway blockade for cancer therapy: Mechanisms, response biomarkers, and combinations. Sci Trans Med (2016) 8(328):328rv4. doi: 10.1126/scitranslmed.aad7118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Li B, Chan HL, Chen P. Immune checkpoint inhibitors: Basics and challenges. Curr medicinal Chem (2019) 26(17):3009–25. doi: 10.2174/0929867324666170804143706 [DOI] [PubMed] [Google Scholar]
- 21. Poggio M, Hu T, Pai CC, Chu B, Belair CD, Chang A, et al. Suppression of exosomal PD-L1 induces systemic anti-tumor immunity and memory. Cell° (2019) 177(2):414–27.e13. doi: 10.1016/j.cell.2019.02.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Thuss-Patience P, Stein A. Immunotherapy in squamous cell cancer of the esophagus. Curr Oncol (Toronto Ont). (2022) 29(4):2461–71. doi: 10.3390/curroncol29040200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Krummel MF, Allison JP. CD28 and CTLA-4 have opposing effects on the response of T cells to stimulation. J Exp Med (1995) 182(2):459–65. doi: 10.1084/jem.182.2.459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ralph C, Elkord E, Burt DJ, O'Dwyer JF, Austin EB, Stern PL, et al. Modulation of lymphocyte regulation for cancer therapy: a phase II trial of tremelimumab in advanced gastric and esophageal adenocarcinoma. Clin Cancer Res an Off J Am Assoc Cancer Res (2010) 16(5):1662–72. doi: 10.1158/1078-0432.CCR-09-2870 [DOI] [PubMed] [Google Scholar]
- 25. Chau I, Doki Y, Ajani JA, Xu J, Wyrwicz L, Motoyama S, et al. Nivolumab (NIVO) plus ipilimumab (IPI) or NIVO plus chemotherapy (chemo) versus chemo as first-line (1L) treatment for advanced esophageal squamous cell carcinoma (ESCC): First results of the CheckMate 648 study. J Clin Oncol (2021) 39(18_suppl):LBA4001–LBA. doi: 10.1200/JCO.2021.39.15_suppl.LBA4001 [DOI] [Google Scholar]
- 26. Wang W, Chen D, Zhao Y, Zhao T, Wen J, Mao Y, et al. Characterization of LAG-3, CTLA-4, and CD8(+) TIL density and their joint influence on the prognosis of patients with esophageal squamous cell carcinoma. Ann Trans Med (2019) 7(23):776. doi: 10.21037/atm.2019.11.38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zheng Y, Li Y, Lian J, Yang H, Li F, Zhao S, et al. TNF-α-induced Tim-3 expression marks the dysfunction of infiltrating natural killer cells in human esophageal cancer. J Trans Med (2019) 17(1):165. doi: 10.1186/s12967-019-1917-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhao JJ, Zhou ZQ, Wang P, Chen CL, Liu Y, Pan QZ, et al. Orchestration of immune checkpoints in tumor immune contexture and their prognostic significance in esophageal squamous cell carcinoma. Cancer Manage Res (2018) 10:6457–68. doi: 10.2147/CMAR.S181949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Koga N, Hu Q, Sakai A, Takada K, Nakanishi R, Hisamatsu Y, et al. Clinical significance of signal regulatory protein alpha (SIRPα) expression in esophageal squamous cell carcinoma. Cancer science. (2021) 112(8):3018–28. doi: 10.1111/cas.14971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yan T, Cui H, Zhou Y, Yang B, Kong P, Zhang Y, et al. Multi-region sequencing unveils novel actionable targets and spatial heterogeneity in esophageal squamous cell carcinoma. Nat Commun (2019) 10(1):1670. doi: 10.1038/s41467-019-09255-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rosenthal R, Cadieux EL, Salgado R, Bakir MA, Moore DA, Hiley CT, et al. Neoantigen-directed immune escape in lung cancer evolution. Nature° (2019) 567(7749):479–85. doi: 10.1038/s41586-019-1032-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zhang H, Xie C, Yue J, Jiang Z, Zhou R, Xie R, et al. Cancer-associated fibroblasts mediated chemoresistance by a FOXO1/TGFβ1 signaling loop in esophageal squamous cell carcinoma. Mol carcinogenesis. (2017) 56(3):1150–63. doi: 10.1002/mc.22581 [DOI] [PubMed] [Google Scholar]
- 33. Higashino N, Koma YI, Hosono M, Takase N, Okamoto M, Kodaira H, et al. Fibroblast activation protein-positive fibroblasts promote tumor progression through secretion of CCL2 and interleukin-6 in esophageal squamous cell carcinoma. Lab investigation; J Tech Methods pathology. (2019) 99(6):777–92. doi: 10.1038/s41374-018-0185-6 [DOI] [PubMed] [Google Scholar]
- 34. Wang T, Niu G, Kortylewski M, Burdelya L, Shain K, Zhang S, et al. Regulation of the innate and adaptive immune responses by stat-3 signaling in tumor cells. Nat Med (2004) 10(1):48–54. doi: 10.1038/nm976 [DOI] [PubMed] [Google Scholar]
- 35. Wang WL, Chang WL, Yang HB, Chang IW, Lee CT, Chang CY, et al. Quantification of tumor infiltrating Foxp3+ regulatory T cells enables the identification of high-risk patients for developing synchronous cancers over upper aerodigestive tract. Oral Oncol (2015) 51(7):698–703. doi: 10.1016/j.oraloncology.2015.04.015 [DOI] [PubMed] [Google Scholar]
- 36. Sahai E, Astsaturov I, Cukierman E, DeNardo DG, Egeblad M, Evans RM, et al. A framework for advancing our understanding of cancer-associated fibroblasts. Nat Rev Cancer. (2020) 20(3):174–86. doi: 10.1038/s41568-019-0238-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kalluri R. The biology and function of fibroblasts in cancer. Nat Rev Cancer. (2016) 16(9):582–98. doi: 10.1038/nrc.2016.73 [DOI] [PubMed] [Google Scholar]
- 38. Kojima Y, Acar A, Eaton EN, Mellody KT, Scheel C, Ben-Porath I, et al. Autocrine TGF-beta and stromal cell-derived factor-1 (SDF-1) signaling drives the evolution of tumor-promoting mammary stromal myofibroblasts. Proc Natl Acad Sci United States America. (2010) 107(46):20009–14. doi: 10.1073/pnas.1013805107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mao X, Xu J, Wang W, Liang C, Hua J, Liu J, et al. Crosstalk between cancer-associated fibroblasts and immune cells in the tumor microenvironment: new findings and future perspectives. Mol cancer. (2021) 20(1):131. doi: 10.1186/s12943-021-01428-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Batlle E, Massagué J. Transforming growth factor-β signaling in immunity and cancer. Immunity° (2019) 50(4):924–40. doi: 10.1016/j.immuni.2019.03.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Freeman P, Mielgo A. Cancer-associated fibroblast mediated inhibition of CD8+ cytotoxic T cell accumulation in tumours: Mechanisms and therapeutic opportunities. Cancers° (2020) 12(9):2687. doi: 10.3390/cancers12092687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ocana A, Nieto-Jiménez C, Pandiella A, Templeton AJ. Neutrophils in cancer: prognostic role and therapeutic strategies. Mol cancer. (2017) 16(1):137. doi: 10.1186/s12943-017-0707-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ksiazkiewicz M, Gottfried E, Kreutz M, Mack M, Hofstaedter F, Kunz-Schughart LA. Importance of CCL2-CCR2A/2B signaling for monocyte migration into spheroids of breast cancer-derived fibroblasts. Immunobiology (2010) 215(9-10):737–47. doi: 10.1016/j.imbio.2010.05.019 [DOI] [PubMed] [Google Scholar]
- 44. Mace TA, Ameen Z, Collins A, Wojcik S, Mair M, Young GS, et al. Pancreatic cancer-associated stellate cells promote differentiation of myeloid-derived suppressor cells in a STAT3-dependent manner. Cancer Res (2013) 73(10):3007–18. doi: 10.1158/0008-5472.CAN-12-4601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Costa A, Kieffer Y, Scholer-Dahirel A, Pelon F, Bourachot B, Cardon M, et al. Fibroblast heterogeneity and immunosuppressive environment in human breast cancer. Cancer Cell (2018) 33(3):463–79.e10. doi: 10.1016/j.ccell.2018.01.011 [DOI] [PubMed] [Google Scholar]
- 46. Lakins MA, Ghorani E, Munir H, Martins CP, Shields JD. Cancer-associated fibroblasts induce antigen-specific deletion of CD8 (+) T cells to protect tumour cells. Nat Commun (2018) 9(1):948. doi: 10.1038/s41467-018-03347-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Qiu L, Yue J, Ding L, Yin Z, Zhang K, Zhang H. Cancer-associated fibroblasts: An emerging target against esophageal squamous cell carcinoma. Cancer letters. (2022) 546:215860. doi: 10.1016/j.canlet.2022.215860 [DOI] [PubMed] [Google Scholar]
- 48. Cui G, Li C, Xu G, Sun Z, Zhu L, Li Z, et al. Tumor-associated fibroblasts and microvessels contribute to the expression of immunosuppressive factor indoleamine 2, 3-dioxygenase in human esophageal cancers. Pathol Oncol Res POR. (2018) 24(2):269–75. doi: 10.1007/s12253-017-0244-0 [DOI] [PubMed] [Google Scholar]
- 49. Zhao Q, Huang L, Qin G, Qiao Y, Ren F, Shen C, et al. Cancer-associated fibroblasts induce monocytic myeloid-derived suppressor cell generation via IL-6/exosomal miR-21-activated STAT3 signaling to promote cisplatin resistance in esophageal squamous cell carcinoma. Cancer letters. (2021) 518:35–48. doi: 10.1016/j.canlet.2021.06.009 [DOI] [PubMed] [Google Scholar]
- 50. Kato T, Noma K, Ohara T, Kashima H, Katsura Y, Sato H, et al. Cancer-associated fibroblasts affect intratumoral CD8(+) and FoxP3(+) T cells Via IL6 in the tumor microenvironment. Clin Cancer Res an Off J Am Assoc Cancer Res (2018) 24(19):4820–33. doi: 10.1158/1078-0432.CCR-18-0205 [DOI] [PubMed] [Google Scholar]
- 51. Huang TX, Tan XY, Huang HS, Li YT, Liu BL, Liu KS, et al. Targeting cancer-associated fibroblast-secreted WNT2 restores dendritic cell-mediated antitumour immunity. Gut° (2022) 71(2):333–44. doi: 10.1136/gutjnl-2020-322924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Chen QY, Li YN, Wang XY, Zhang X, Hu Y, Li L, et al. Tumor fibroblast-derived FGF2 regulates expression of SPRY1 in esophageal tumor-infiltrating T cells and plays a role in T-cell exhaustion. Cancer Res (2020) 80(24):5583–96. doi: 10.1158/0008-5472.CAN-20-1542 [DOI] [PubMed] [Google Scholar]
- 53. Chen Y, McAndrews KM, Kalluri R. Clinical and therapeutic relevance of cancer-associated fibroblasts. Nat Rev Clin Oncol (2021) 18(12):792–804. doi: 10.1038/s41571-021-00546-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Zhen Z, Tang W, Wang M, Zhou S, Wang H, Wu Z, et al. Protein nanocage mediated fibroblast-activation protein targeted photoimmunotherapy to enhance cytotoxic T cell infiltration and tumor control. Nano letters. (2017) 17(2):862–9. doi: 10.1021/acs.nanolett.6b04150 [DOI] [PubMed] [Google Scholar]
- 55. Duperret EK, Trautz A, Ammons D, Perales-Puchalt A, Wise MC, Yan J, et al. Alteration of the tumor stroma using a consensus DNA vaccine targeting fibroblast activation protein (FAP) synergizes with antitumor vaccine therapy in mice. Clin Cancer Res an Off J Am Assoc Cancer Res (2018) 24(5):1190–201. doi: 10.1158/1078-0432.CCR-17-2033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. de Sostoa J, Fajardo CA, Moreno R, Ramos MD, Farrera-Sal M, Alemany R. Targeting the tumor stroma with an oncolytic adenovirus secreting a fibroblast activation protein-targeted bispecific T-cell engager. J immunotherapy cancer. (2019) 7(1):19. doi: 10.1186/s40425-019-0505-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell° (2011) 144(5):646–74. doi: 10.1016/j.cell.2011.02.013 [DOI] [PubMed] [Google Scholar]
- 58. Ostuni R, Kratochvill F, Murray PJ, Natoli G. Macrophages and cancer: from mechanisms to therapeutic implications. Trends Immunol (2015) 36(4):229–39. doi: 10.1016/j.it.2015.02.004 [DOI] [PubMed] [Google Scholar]
- 59. Condeelis J, Pollard JW. Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell° (2006) 124(2):263–6. doi: 10.1016/j.cell.2006.01.007 [DOI] [PubMed] [Google Scholar]
- 60. Gao J, Liang Y, Wang L. Shaping polarization of tumor-associated macrophages in cancer immunotherapy. Front Immunol (2022) 13:888713. doi: 10.3389/fimmu.2022.888713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Balkwill F. Cancer and the chemokine network. Nat Rev Cancer. (2004) 4(7):540–50. doi: 10.1038/nrc1388 [DOI] [PubMed] [Google Scholar]
- 62. Gordon SR, Maute RL, Dulken BW, Hutter G, George BM, McCracken MN, et al. PD-1 expression by tumour-associated macrophages inhibits phagocytosis and tumour immunity. Nature° (2017) 545(7655):495–9. doi: 10.1038/nature22396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Zheng Y, Chen Z, Han Y, Han L, Zou X, Zhou B, et al. Immune suppressive landscape in the human esophageal squamous cell carcinoma microenvironment. Nat Commun (2020) 11(1):6268. doi: 10.1038/s41467-020-20019-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Yagi T, Baba Y, Okadome K, Kiyozumi Y, Hiyoshi Y, Ishimoto T, et al. Tumour-associated macrophages are associated with poor prognosis and programmed death ligand 1 expression in oesophageal cancer. Eur J Cancer (Oxford Engl 1990). (2019) 111:38–49. doi: 10.1016/j.ejca.2019.01.018 [DOI] [PubMed] [Google Scholar]
- 65. Murray PJ, Wynn TA. Protective and pathogenic functions of macrophage subsets. Nat Rev Immunol (2011) 11(11):723–37. doi: 10.1038/nri3073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Yang H, Zhang Q, Xu M, Wang L, Chen X, Feng Y, et al. CCL2-CCR2 axis recruits tumor associated macrophages to induce immune evasion through PD-1 signaling in esophageal carcinogenesis. Mol cancer. (2020) 19(1):41. doi: 10.1186/s12943-020-01165-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Fujiwara T, Yakoub MA, Chandler A, Christ AB, Yang G, Ouerfelli O, et al. CSF1/CSF1R signaling inhibitor pexidartinib (PLX3397) reprograms tumor-associated macrophages and stimulates T-cell infiltration in the sarcoma microenvironment. Mol Cancer Ther (2021) 20(8):1388–99. doi: 10.1158/1535-7163.MCT-20-0591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Omstead AN, Paskewicz M, Gorbunova A, Zheng P, Salvitti MS, Mansoor R, et al. CSF-1R inhibitor, pexidartinib, sensitizes esophageal adenocarcinoma to PD-1 immune checkpoint blockade in a rat model. Carcinogenesis (2022) 43(9):842–50. doi: 10.1093/carcin/bgac043 [DOI] [PubMed] [Google Scholar]
- 69. Argyle D, Kitamura T. Targeting macrophage-recruiting chemokines as a novel therapeutic strategy to prevent the progression of solid tumors. Front Immunol (2018) 9:2629. doi: 10.3389/fimmu.2018.02629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Pyonteck SM, Akkari L, Schuhmacher AJ, Bowman RL, Sevenich L, Quail DF, et al. CSF-1R inhibition alters macrophage polarization and blocks glioma progression. Nat Med (2013) 19(10):1264–72. doi: 10.1038/nm.3337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Brana I, Calles A, LoRusso PM, Yee LK, Puchalski TA, Seetharam S, et al. Carlumab, an anti-C-C chemokine ligand 2 monoclonal antibody, in combination with four chemotherapy regimens for the treatment of patients with solid tumors: an open-label, multicenter phase 1b study. Targeted Oncol (2015) 10(1):111–23. doi: 10.1007/s11523-014-0320-2 [DOI] [PubMed] [Google Scholar]
- 72. Razak AR, Cleary JM, Moreno V, Boyer M, Calvo Aller E, Edenfield W, et al. Safety and efficacy of AMG 820, an anti-colony-stimulating factor 1 receptor antibody, in combination with pembrolizumab in adults with advanced solid tumors. J immunotherapy Cancer (2020) 8(2):e001006. doi: 10.1136/jitc-2020-001006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Tanaka A, Sakaguchi S. Targeting treg cells in cancer immunotherapy. Eur J Immunol (2019) 49(8):1140–6. doi: 10.1002/eji.201847659 [DOI] [PubMed] [Google Scholar]
- 74. Tie Y, Tang F, Wei YQ, Wei XW. Immunosuppressive cells in cancer: mechanisms and potential therapeutic targets. J Hematol Oncol (2022) 15(1):61. doi: 10.1186/s13045-022-01282-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Saraiva M, Vieira P, O'Garra A. Biology and therapeutic potential of interleukin-10. J Exp Med (2020) 217(1):e2019041. doi: 10.1084/jem.20190418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Tanaka A, Sakaguchi S. Regulatory T cells in cancer immunotherapy. Cell Res (2017) 27(1):109–18. doi: 10.1038/cr.2016.151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Yazdani Z, Rafiei A, Golpour M, Zafari P, Moonesi M, Ghaffari S. IL-35, a double-edged sword in cancer. J Cell Biochem (2020) 121(3):2064–76. doi: 10.1002/jcb.29441 [DOI] [PubMed] [Google Scholar]
- 78. Tekguc M, Wing JB, Osaki M, Long J, Sakaguchi S. Treg-expressed CTLA-4 depletes CD80/CD86 by trogocytosis, releasing free PD-L1 on antigen-presenting cells. Proc Natl Acad Sci United States America (2021) 118(30):e2023739118. doi: 10.1073/pnas.2023739118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Sun B, Liu M, Cui M, Li T. Granzyme b-expressing treg cells are enriched in colorectal cancer and present the potential to eliminate autologous T conventional cells. Immunol letters. (2020) 217:7–14. doi: 10.1016/j.imlet.2019.10.007 [DOI] [PubMed] [Google Scholar]
- 80. Ichihara F, Kono K, Takahashi A, Kawaida H, Sugai H, Fujii H. Increased populations of regulatory T cells in peripheral blood and tumor-infiltrating lymphocytes in patients with gastric and esophageal cancers. Clin Cancer Res an Off J Am Assoc Cancer Res (2003) 9(12):4404–8. [PubMed] [Google Scholar]
- 81. Lin EW, Karakasheva TA, Hicks PD, Bass AJ, Rustgi AK. The tumor microenvironment in esophageal cancer. Oncogene° (2016) 35(41):5337–49. doi: 10.1038/onc.2016.34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Nabeki B, Ishigami S, Uchikado Y, Sasaki K, Kita Y, Okumura H, et al. Interleukin-32 expression and treg infiltration in esophageal squamous cell carcinoma. Anticancer Res (2015) 35(5):2941–7. [PubMed] [Google Scholar]
- 83. Yue Y, Lian J, Wang T, Luo C, Yuan Y, Qin G, et al. Interleukin-33-nuclear factor-κB-CCL2 signaling pathway promotes progression of esophageal squamous cell carcinoma by directing regulatory T cells. Cancer science. (2020) 111(3):795–806. doi: 10.1111/cas.14293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Zhao X, Liu S, Chen X, Zhao J, Li F, Zhao Q, et al. L1CAM overexpression promotes tumor progression through recruitment of regulatory T cells in esophageal carcinoma. Cancer Biol Med (2021) 18(2):547–61. doi: 10.20892/j.issn.2095-3941.2020.0182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Han L, Chen S, Chen Z, Zhou B, Zheng Y, Shen L. Interleukin 32 promotes Foxp3(+) treg cell development and CD8(+) T cell function in human esophageal squamous cell carcinoma microenvironment. Front Cell Dev Biol (2021) 9:704853. doi: 10.3389/fcell.2021.704853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Kumar V, Patel S, Tcyganov E, Gabrilovich DI. The nature of myeloid-derived suppressor cells in the tumor microenvironment. Trends Immunol (2016) 37(3):208–20. doi: 10.1016/j.it.2016.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol (2009) 9(3):162–74. doi: 10.1038/nri2506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Groth C, Hu X, Weber R, Fleming V, Altevogt P, Utikal J, et al. Immunosuppression mediated by myeloid-derived suppressor cells (MDSCs) during tumour progression. Br J cancer. (2019) 120(1):16–25. doi: 10.1038/s41416-018-0333-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Serafini P, Mgebroff S, Noonan K, Borrello I. Myeloid-derived suppressor cells promote cross-tolerance in b-cell lymphoma by expanding regulatory T cells. Cancer Res (2008) 68(13):5439–49. doi: 10.1158/0008-5472.CAN-07-6621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Gabitass RF, Annels NE, Stocken DD, Pandha HA, Middleton GW. Elevated myeloid-derived suppressor cells in pancreatic, esophageal and gastric cancer are an independent prognostic factor and are associated with significant elevation of the Th2 cytokine interleukin-13. Cancer immunology immunotherapy CII. (2011) 60(10):1419–30. doi: 10.1007/s00262-011-1028-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Huang H, Zhang G, Li G, Ma H, Zhang X. Circulating CD14(+)HLA-DR(-/low) myeloid-derived suppressor cell is an indicator of poor prognosis in patients with ESCC. Tumour Biol J Int Soc Oncodevelopmental Biol Med (2015) 36(10):7987–96. doi: 10.1007/s13277-015-3426-y [DOI] [PubMed] [Google Scholar]
- 92. Chen MF, Kuan FC, Yen TC, Lu MS, Lin PY, Chung YH, et al. IL-6-stimulated CD11b+ CD14+ HLA-DR- myeloid-derived suppressor cells, are associated with progression and poor prognosis in squamous cell carcinoma of the esophagus. Oncotarget° (2014) 5(18):8716–28. doi: 10.18632/oncotarget.2368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Karakasheva TA, Waldron TJ, Eruslanov E, Kim SB, Lee JS, O'Brien S, et al. CD38-expressing myeloid-derived suppressor cells promote tumor growth in a murine model of esophageal cancer. Cancer Res (2015) 75(19):4074–85. doi: 10.1158/0008-5472.CAN-14-3639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Li P, Chen X, Qin G, Yue D, Zhang Z, Ping Y, et al. Maelstrom directs myeloid-derived suppressor cells to promote esophageal squamous cell carcinoma progression via activation of the Akt1/RelA/IL8 signaling pathway. Cancer Immunol Res (2018) 6(10):1246–59. doi: 10.1158/2326-6066.CIR-17-0415 [DOI] [PubMed] [Google Scholar]
- 95. Yue D, Liu S, Zhang T, Wang Y, Qin G, Chen X, et al. NEDD9 promotes cancer stemness by recruiting myeloid-derived suppressor cells via CXCL8 in esophageal squamous cell carcinoma. Cancer Biol Med (2021) 18(3):705–20. doi: 10.20892/j.issn.2095-3941.2020.0290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Zhang X, Zhang W, Yuan X, Fu M, Qian H, Xu W. Neutrophils in cancer development and progression: Roles, mechanisms, and implications (Review). Int J Oncol (2016) 49(3):857–67. doi: 10.3892/ijo.2016.3616 [DOI] [PubMed] [Google Scholar]
- 97. Zhang Y, Guoqiang L, Sun M, Lu X. Targeting and exploitation of tumor-associated neutrophils to enhance immunotherapy and drug delivery for cancer treatment. Cancer Biol Med (2020) 17(1):32–43. doi: 10.20892/j.issn.2095-3941.2019.0372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Fridlender ZG, Sun J, Kim S, Kapoor V, Cheng G, Ling L, et al. Polarization of tumor-associated neutrophil phenotype by TGF-beta: "N1" versus "N2" TAN. Cancer Cell (2009) 16(3):183–94. doi: 10.1016/j.ccr.2009.06.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Sun Y, Zhang L. The clinical use of pretreatment NLR, PLR, and LMR in patients with esophageal squamous cell carcinoma: evidence from a meta-analysis. Cancer Manage Res (2018) 10:6167–79. doi: 10.2147/CMAR.S171035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Ishibashi Y, Tsujimoto H, Einama T, Mochizuki S, Kouzu K, Nomura S, et al. Correlation between immunoinflammatory measures and periostin expression in esophageal squamous cell carcinoma: A single-center, retrospective cohort study. Ann Surg Oncol (2021) 28(2):1228–37. doi: 10.1245/s10434-020-08765-3 [DOI] [PubMed] [Google Scholar]
- 101. Guthrie GJ, Charles KA, Roxburgh CS, Horgan PG, McMillan DC, Clarke SJ. The systemic inflammation-based neutrophil-lymphocyte ratio: experience in patients with cancer. Crit Rev oncology/hematology. (2013) 88(1):218–30. doi: 10.1016/j.critrevonc.2013.03.010 [DOI] [PubMed] [Google Scholar]
- 102. Papayannopoulos V. Neutrophil extracellular traps in immunity and disease. Nat Rev Immunol (2018) 18(2):134–47. doi: 10.1038/nri.2017.105 [DOI] [PubMed] [Google Scholar]
- 103. Masucci MT, Minopoli M, Del Vecchio S, Carriero MV. The emerging role of neutrophil extracellular traps (NETs) in tumor progression and metastasis. Front Immunol (2020) 11:1749. doi: 10.3389/fimmu.2020.01749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Zhang H, Lv H, Weng M, Wang H, Cata JP, Chen W, et al. Preoperative leukocytosis is associated with increased tumor-infiltrating neutrophil extracellular traps and worse outcomes in esophageal cancer. Ann Trans Med (2020) 8(7):441. doi: 10.21037/atm.2020.03.190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Chen CL, Wang Y, Huang CY, Zhou ZQ, Zhao JJ, Zhang XF, et al. IL-17 induces antitumor immunity by promoting beneficial neutrophil recruitment and activation in esophageal squamous cell carcinoma. Oncoimmunology° (2017) 7(1):e1373234. doi: 10.1080/2162402X.2017.1373234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Que H, Fu Q, Lan T, Tian X, Wei X. Tumor-associated neutrophils and neutrophil-targeted cancer therapies. Biochim Biophys Acta Rev cancer. (2022) 1877(5):188762. doi: 10.1016/j.bbcan.2022.188762 [DOI] [PubMed] [Google Scholar]
- 107. Schott AF, Goldstein LJ, Cristofanilli M, Ruffini PA, McCanna S, Reuben JM, et al. Phase ib pilot study to evaluate reparixin in combination with weekly paclitaxel in patients with HER-2-Negative metastatic breast cancer. Clin Cancer Res an Off J Am Assoc Cancer Res (2017) 23(18):5358–65. doi: 10.1158/1078-0432.CCR-16-2748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Tazzyman S, Barry ST, Ashton S, Wood P, Blakey D, Lewis CE, et al. Inhibition of neutrophil infiltration into A549 lung tumors in vitro and in vivo using a CXCR2-specific antagonist is associated with reduced tumor growth. Int J cancer. (2011) 129(4):847–58. doi: 10.1002/ijc.25987 [DOI] [PubMed] [Google Scholar]
- 109. Holz O, Khalilieh S, Ludwig-Sengpiel A, Watz H, Stryszak P, Soni P, et al. SCH527123, a novel CXCR2 antagonist, inhibits ozone-induced neutrophilia in healthy subjects. Eur Respir J (2010) 35(3):564–70. doi: 10.1183/09031936.00048509 [DOI] [PubMed] [Google Scholar]
- 110. Yasuda K, Takeuchi Y, Hirota K. The pathogenicity of Th17 cells in autoimmune diseases. Semin immunopathology. (2019) 41(3):283–97. doi: 10.1007/s00281-019-00733-8 [DOI] [PubMed] [Google Scholar]
- 111. Lu L, Pan K, Zheng HX, Li JJ, Qiu HJ, Zhao JJ, et al. IL-17A promotes immune cell recruitment in human esophageal cancers and the infiltrating dendritic cells represent a positive prognostic marker for patient survival. J Immunotherapy (Hagerstown Md 1997). (2013) 36(8):451–8. doi: 10.1097/CJI.0b013e3182a802cf [DOI] [PubMed] [Google Scholar]
- 112. Lu L, Weng C, Mao H, Fang X, Liu X, Wu Y, et al. IL-17A promotes migration and tumor killing capability of b cells in esophageal squamous cell carcinoma. Oncotarget° (2016) 7(16):21853–64. doi: 10.18632/oncotarget.7869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Chen D, Hu Q, Mao C, Jiao Z, Wang S, Yu L, et al. Increased IL-17-producing CD4(+) T cells in patients with esophageal cancer. Cell Immunol (2012) 272(2):166–74. doi: 10.1016/j.cellimm.2011.10.015 [DOI] [PubMed] [Google Scholar]
- 114. Gomez-Rodriguez J, Wohlfert EA, Handon R, Meylan F, Wu JZ, Anderson SM, et al. Itk-mediated integration of T cell receptor and cytokine signaling regulates the balance between Th17 and regulatory T cells. J Exp Med (2014) 211(3):529–43. doi: 10.1084/jem.20131459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Borish LC, Steinke JW. 2. cytokines and chemokines. J Allergy Clin Immunol (2003) 111(2 Suppl):S460–75. doi: 10.1067/mai.2003.108 [DOI] [PubMed] [Google Scholar]
- 116. Li L, Yu R, Cai T, Chen Z, Lan M, Zou T, et al. Effects of immune cells and cytokines on inflammation and immunosuppression in the tumor microenvironment. Int immunopharmacology. (2020) 88:106939. doi: 10.1016/j.intimp.2020.106939 [DOI] [PubMed] [Google Scholar]
- 117. Zhao H, Wei J, Sun J. Roles of TGF-β signaling pathway in tumor microenvirionment and cancer therapy. Int Immunopharmacol (2020) 89(Pt B):107101. doi: 10.1016/j.intimp.2020.107101 [DOI] [PubMed] [Google Scholar]
- 118. Yang L, Pang Y, Moses HL. TGF-beta and immune cells: an important regulatory axis in the tumor microenvironment and progression. Trends Immunol (2010) 31(6):220–7. doi: 10.1016/j.it.2010.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Garcia-Rendueles AR, Rodrigues JS, Garcia-Rendueles ME, Suarez-Fariña M, Perez-Romero S, Barreiro F, et al. Rewiring of the apoptotic TGF-β-SMAD/NFκB pathway through an oncogenic function of p27 in human papillary thyroid cancer. Oncogene° (2017) 36(5):652–66. doi: 10.1038/onc.2016.233 [DOI] [PubMed] [Google Scholar]
- 120. Fang P, Zhou J, Liang Z, Yang Y, Luan S, Xiao X, et al. Immunotherapy resistance in esophageal cancer: Possible mechanisms and clinical implications. Front Immunol (2022) 13:975986. doi: 10.3389/fimmu.2022.975986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Fiori ME, Di Franco S, Villanova L, Bianca P, Stassi G, De Maria R. Cancer-associated fibroblasts as abettors of tumor progression at the crossroads of EMT and therapy resistance. Mol cancer. (2019) 18(1):70. doi: 10.1186/s12943-019-0994-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Flavell RA, Sanjabi S, Wrzesinski SH, Licona-Limón P. The polarization of immune cells in the tumour environment by TGFbeta. Nat Rev Immunol (2010) 10(8):554–67. doi: 10.1038/nri2808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Blum AE, Venkitachalam S, Ravillah D, Chelluboyina AK, Kieber-Emmons AM, Ravi L, et al. Systems biology analyses show hyperactivation of transforming growth factor-β and JNK signaling pathways in esophageal cancer. Gastroenterology° (2019) 156(6):1761–74. doi: 10.1053/j.gastro.2019.01.263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Gholamin M, Moaven O, Memar B, Farshchian M, Naseh H, Malekzadeh R, et al. Overexpression and interactions of interleukin-10, transforming growth factor beta, and vascular endothelial growth factor in esophageal squamous cell carcinoma. World J surgery. (2009) 33(7):1439–45. doi: 10.1007/s00268-009-0070-y [DOI] [PubMed] [Google Scholar]
- 125. Li Y, An J, Huang S, He J, Zhang J. Esophageal cancer-derived microvesicles induce regulatory b cells. Cell Biochem Funct (2015) 33(5):308–13. doi: 10.1002/cbf.3115 [DOI] [PubMed] [Google Scholar]
- 126. Chen X, Wang L, Li P, Song M, Qin G, Gao Q, et al. Dual TGF-β and PD-1 blockade synergistically enhances MAGE-A3-specific CD8(+) T cell response in esophageal squamous cell carcinoma. Int J cancer. (2018) 143(10):2561–74. doi: 10.1002/ijc.31730 [DOI] [PubMed] [Google Scholar]
- 127. Strauss J, Heery CR, Schlom J, Madan RA, Cao L, Kang Z, et al. Phase I trial of M7824 (MSB0011359C), a bifunctional fusion protein targeting PD-L1 and TGFβ, in advanced solid tumors. Clin Cancer Res an Off J Am Assoc Cancer Res (2018) 24(6):1287–95. doi: 10.1158/1078-0432.CCR-17-2653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Lan Y, Zhang D, Xu C, Hance KW, Marelli B, Qi J, et al. Enhanced preclinical antitumor activity of M7824, a bifunctional fusion protein simultaneously targeting PD-L1 and TGF-β. Sci Trans Med (2018) 10(424):eaan5488. doi: 10.1126/scitranslmed.aan5488 [DOI] [PubMed] [Google Scholar]
- 129. Lippitz BE. Cytokine patterns in patients with cancer: a systematic review. Lancet Oncol (2013) 14(6):e218–28. doi: 10.1016/S1470-2045(12)70582-X [DOI] [PubMed] [Google Scholar]
- 130. Hodge DR, Hurt EM, Farrar WL. The role of IL-6 and STAT3 in inflammation and cancer. Eur J Cancer (Oxford Engl 1990). (2005) 41(16):2502–12. doi: 10.1016/j.ejca.2005.08.016 [DOI] [PubMed] [Google Scholar]
- 131. Chen MF, Chen PT, Lu MS, Lin PY, Chen WC, Lee KD. IL-6 expression predicts treatment response and outcome in squamous cell carcinoma of the esophagus. Mol cancer. (2013) 12:26. doi: 10.1186/1476-4598-12-26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Chen X, Wang L, Wang W, Zhao L, Shan B. B7-H4 facilitates proliferation of esophageal squamous cell carcinoma cells through promoting interleukin-6/signal transducer and activator of transcription 3 pathway activation. Cancer science. (2016) 107(7):944–54. doi: 10.1111/cas.12949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Berraondo P, Sanmamed MF, Ochoa MC, Etxeberria I, Aznar MA, Pérez-Gracia JL, et al. Cytokines in clinical cancer immunotherapy. Br J cancer. (2019) 120(1):6–15. doi: 10.1038/s41416-018-0328-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Miller MC, Mayo KH. Chemokines from a structural perspective. Int J Mol Sci (2017) 18(10):2088. doi: 10.3390/ijms18102088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Bule P, Aguiar SI, Aires-Da-Silva F, Dias JNR. Chemokine-directed tumor microenvironment modulation in cancer immunotherapy. Int J Mol Sci (2021) 22(18):9804. doi: 10.3390/ijms22189804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Liu JY, Li F, Wang LP, Chen XF, Wang D, Cao L, et al. CTL- vs treg lymphocyte-attracting chemokines, CCL4 and CCL20, are strong reciprocal predictive markers for survival of patients with oesophageal squamous cell carcinoma. Br J cancer. (2015) 113(5):747–55. doi: 10.1038/bjc.2015.290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Chen D, Jiang R, Mao C, Shi L, Wang S, Yu L, et al. Chemokine/chemokine receptor interactions contribute to the accumulation of Th17 cells in patients with esophageal squamous cell carcinoma. Hum Immunol (2012) 73(11):1068–72. doi: 10.1016/j.humimm.2012.07.333 [DOI] [PubMed] [Google Scholar]
- 138. Märkl F, Huynh D, Endres S, Kobold S. Utilizing chemokines in cancer immunotherapy. Trends cancer. (2022) 8(8):670–82. doi: 10.1016/j.trecan.2022.04.001 [DOI] [PubMed] [Google Scholar]
- 139. Qian BZ, Li J, Zhang H, Kitamura T, Zhang J, Campion LR, et al. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature° (2011) 475(7355):222–5. doi: 10.1038/nature10138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Dominguez C, McCampbell KK, David JM, Palena C. Neutralization of IL-8 decreases tumor PMN-MDSCs and reduces mesenchymalization of claudin-low triple-negative breast cancer. JCI Insight (2017) 2(21):e94296. doi: 10.1172/jci.insight.94296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Bilusic M, Heery CR, Collins JM, Donahue RN, Palena C, Madan RA, et al. Phase I trial of HuMax-IL8 (BMS-986253), an anti-IL-8 monoclonal antibody, in patients with metastatic or unresectable solid tumors. J immunotherapy cancer. (2019) 7(1):240. doi: 10.1186/s40425-019-0706-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Han P, Cao P, Hu S, Kong K, Deng Y, Zhao B, et al. Esophageal microenvironment: From precursor microenvironment to premetastatic niche. Cancer Manage Res (2020) 12:5857–79. doi: 10.2147/CMAR.S258215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Kunz M, Ibrahim SM. Molecular responses to hypoxia in tumor cells. Mol cancer. (2003) 2:23. doi: 10.1186/1476-4598-2-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Kizaka-Kondoh S, Tanaka S, Harada H, Hiraoka M. The HIF-1-active microenvironment: an environmental target for cancer therapy. Advanced Drug delivery Rev (2009) 61(7-8):623–32. doi: 10.1016/j.addr.2009.01.006 [DOI] [PubMed] [Google Scholar]
- 145. Jin J, Zhao Q. Emerging role of mTOR in tumor immune contexture: Impact on chemokine-related immune cells migration. Theranostics° (2020) 10(14):6231–44. doi: 10.7150/thno.45219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Germain S, Monnot C, Muller L, Eichmann A. Hypoxia-driven angiogenesis: role of tip cells and extracellular matrix scaffolding. Curr Opin hematology. (2010) 17(3):245–51. doi: 10.1097/MOH.0b013e32833865b9 [DOI] [PubMed] [Google Scholar]
- 147. Gilkes DM, Semenza GL, Wirtz D. Hypoxia and the extracellular matrix: drivers of tumour metastasis. Nat Rev Cancer. (2014) 14(6):430–9. doi: 10.1038/nrc3726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Damgaci S, Ibrahim-Hashim A, Enriquez-Navas PM, Pilon-Thomas S, Guvenis A, Gillies RJ. Hypoxia and acidosis: immune suppressors and therapeutic targets. Immunology° (2018) 154(3):354–62. doi: 10.1111/imm.12917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Lin A, Maity A. Molecular pathways: A novel approach to targeting hypoxia and improving radiotherapy efficacy via reduction in oxygen demand. Clin Cancer Res an Off J Am Assoc Cancer Res (2015) 21(9):1995–2000. doi: 10.1158/1078-0432.CCR-14-0858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Luan S, Zeng X, Zhang C, Qiu J, Yang Y, Mao C, et al. Advances in drug resistance of esophageal cancer: From the perspective of tumor microenvironment. Front Cell Dev Biol (2021) 9:664816. doi: 10.3389/fcell.2021.664816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Augustin RC, Delgoffe GM, Najjar YG. Characteristics of the tumor microenvironment that influence immune cell functions: Hypoxia, oxidative stress, metabolic alterations. Cancers° (2020) 12(12):3802. doi: 10.3390/cancers12123802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Vito A, El-Sayes N, Mossman K. Hypoxia-driven immune escape in the tumor microenvironment. Cells° (2020) 9(4):992. doi: 10.3390/cells9040992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Mortezaee K. Immune escape: A critical hallmark in solid tumors. Life Sci (2020) 258:118110. doi: 10.1016/j.lfs.2020.118110 [DOI] [PubMed] [Google Scholar]
- 154. Sormendi S, Wielockx B. Hypoxia pathway proteins as central mediators of metabolism in the tumor cells and their microenvironment. Front Immunol (2018) 9:40. doi: 10.3389/fimmu.2018.00040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Kimura S, Kitadai Y, Tanaka S, Kuwai T, Hihara J, Yoshida K, et al. Expression of hypoxia-inducible factor (HIF)-1alpha is associated with vascular endothelial growth factor expression and tumour angiogenesis in human oesophageal squamous cell carcinoma. Eur J Cancer (Oxford Engl 1990). (2004) 40(12):1904–12. doi: 10.1016/j.ejca.2004.04.035 [DOI] [PubMed] [Google Scholar]
- 156. Lu Q, Wang X, Zhu J, Fei X, Chen H, Li C. Hypoxic tumor-derived exosomal Circ0048117 facilitates M2 macrophage polarization acting as miR-140 sponge in esophageal squamous cell carcinoma. OncoTargets Ther (2020) 13:11883–97. doi: 10.2147/OTT.S284192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. He Z, Liu H, Guan H, Ji J, Jiang Y, Zhang N, et al. Construction of a prognostic model for hypoxia-related LncRNAs and prediction of the immune landscape in the digestive system pan-cancer. Front Oncol (2022) 12:812786. doi: 10.3389/fonc.2022.812786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Colegio OR, Chu NQ, Szabo AL, Chu T, Rhebergen AM, Jairam V, et al. Functional polarization of tumour-associated macrophages by tumour-derived lactic acid. Nature° (2014) 513(7519):559–63. doi: 10.1038/nature13490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Díaz FE, Dantas E, Cabrera M, Benítez CA, Delpino MV, Duette G, et al. Fever-range hyperthermia improves the anti-apoptotic effect induced by low pH on human neutrophils promoting a proangiogenic profile. Cell Death disease. (2016) 7(10):e2437. doi: 10.1038/cddis.2016.337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Pötzl J, Roser D, Bankel L, Hömberg N, Geishauser A, Brenner CD, et al. Reversal of tumor acidosis by systemic buffering reactivates NK cells to express IFN-γ and induces NK cell-dependent lymphoma control without other immunotherapies. Int J cancer. (2017) 140(9):2125–33. doi: 10.1002/ijc.30646 [DOI] [PubMed] [Google Scholar]
- 161. Calcinotto A, Filipazzi P, Grioni M, Iero M, De Milito A, Ricupito A, et al. Modulation of microenvironment acidity reverses anergy in human and murine tumor-infiltrating T lymphocytes. Cancer Res (2012) 72(11):2746–56. doi: 10.1158/0008-5472.CAN-11-1272 [DOI] [PubMed] [Google Scholar]
- 162. Luo Y, Li L, Chen X, Gou H, Yan K, Xu Y. Effects of lactate in immunosuppression and inflammation: Progress and prospects. Int Rev Immunol (2022) 41(1):19–29. doi: 10.1080/08830185.2021.1974856 [DOI] [PubMed] [Google Scholar]
- 163. Kumagai S, Koyama S, Itahashi K, Tanegashima T, Lin YT, Togashi Y, et al. Lactic acid promotes PD-1 expression in regulatory T cells in highly glycolytic tumor microenvironments. Cancer Cell (2022) 40(2):201–18.e9. doi: 10.1016/j.ccell.2022.01.001 [DOI] [PubMed] [Google Scholar]
- 164. Drenckhan A, Freytag M, Supuran CT, Sauter G, Izbicki JR, Gros SJ. CAIX furthers tumour progression in the hypoxic tumour microenvironment of esophageal carcinoma and is a possible therapeutic target. J Enzyme inhibition medicinal Chem (2018) 33(1):1024–33. doi: 10.1080/14756366.2018.1475369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Jomrich G, Jesch B, Birner P, Schwameis K, Paireder M, Asari R, et al. Stromal expression of carbonic anhydrase IX in esophageal cancer. Clin Trans Oncol Off Publ Fed Spanish Oncol Societies Natl Cancer Institute Mexico. (2014) 16(11):966–72. doi: 10.1007/s12094-014-1180-z [DOI] [PubMed] [Google Scholar]
- 166. McGettrick AF, O'Neill LAJ. The role of HIF in immunity and inflammation. Cell Metab (2020) 32(4):524–36. doi: 10.1016/j.cmet.2020.08.002 [DOI] [PubMed] [Google Scholar]
- 167. Ananthakrishnan AN, Bernstein CN, Iliopoulos D, Macpherson A, Neurath MF, Ali RAR, et al. Environmental triggers in IBD: a review of progress and evidence. Nat Rev Gastroenterol hepatology. (2018) 15(1):39–49. doi: 10.1038/nrgastro.2017.136 [DOI] [PubMed] [Google Scholar]
- 168. Holohan C, Van Schaeybroeck S, Longley DB, Johnston PG. Cancer drug resistance: an evolving paradigm. Nat Rev Cancer. (2013) 13(10):714–26. doi: 10.1038/nrc3599 [DOI] [PubMed] [Google Scholar]
- 169. Trédan O, Galmarini CM, Patel K, Tannock IF. Drug resistance and the solid tumor microenvironment. J Natl Cancer Institute. (2007) 99(19):1441–54. doi: 10.1093/jnci/djm135 [DOI] [PubMed] [Google Scholar]
- 170. Annese T, Tamma R, Ribatti D. Update in TIGIT immune-checkpoint role in cancer. Front Oncol (2022) 12:871085. doi: 10.3389/fonc.2022.871085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Tian T, Li Z. Targeting Tim-3 in cancer with resistance to PD-1/PD-L1 blockade. Front Oncol (2021) 11:731175. doi: 10.3389/fonc.2021.731175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Schumacher TN, Schreiber RD. Neoantigens in cancer immunotherapy. Sci (New York NY). (2015) 348(6230):69–74. doi: 10.1126/science.aaa4971 [DOI] [PubMed] [Google Scholar]
- 173. Gettinger S, Choi J, Hastings K, Truini A, Datar I, Sowell R, et al. Impaired HLA class I antigen processing and presentation as a mechanism of acquired resistance to immune checkpoint inhibitors in lung cancer. Cancer discovery. (2017) 7(12):1420–35. doi: 10.1158/2159-8290.CD-17-0593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Schoenfeld AJ, Hellmann MD. Acquired resistance to immune checkpoint inhibitors. Cancer Cell (2020) 37(4):443–55. doi: 10.1016/j.ccell.2020.03.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. McGranahan N, Furness AJ, Rosenthal R, Ramskov S, Lyngaa R, Saini SK, et al. Clonal neoantigens elicit T cell immunoreactivity and sensitivity to immune checkpoint blockade. Sci (New York NY). (2016) 351(6280):1463–9. doi: 10.1126/science.aaf1490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Doki Y, Ajani JA, Kato K, Xu J, Wyrwicz L, Motoyama S, et al. Nivolumab combination therapy in advanced esophageal squamous-cell carcinoma. New Engl J Med (2022) 386(5):449–62. doi: 10.1056/NEJMoa2111380 [DOI] [PubMed] [Google Scholar]
- 177. Luo H, Lu J, Bai Y, Mao T, Wang J, Fan Q, et al. Effect of camrelizumab vs placebo added to chemotherapy on survival and progression-free survival in patients with advanced or metastatic esophageal squamous cell carcinoma: The ESCORT-1st randomized clinical trial. Jama° (2021) 326(10):916–25. doi: 10.1001/jama.2021.12836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Wang ZX, Cui C, Yao J, Zhang Y, Li M, Feng J, et al. Toripalimab plus chemotherapy in treatment-naïve, advanced esophageal squamous cell carcinoma (JUPITER-06): A multi-center phase 3 trial. Cancer Cell (2022) 40(3):277–88.e3. doi: 10.1016/j.ccell.2022.02.007 [DOI] [PubMed] [Google Scholar]
- 179. Lu Z, Wang J, Shu Y, Liu L, Kong L, Yang L, et al. Sintilimab versus placebo in combination with chemotherapy as first line treatment for locally advanced or metastatic oesophageal squamous cell carcinoma (ORIENT-15): multicentre, randomised, double blind, phase 3 trial. BMJ (Clinical Res ed) (2022) 377:e068714. doi: 10.1136/bmj-2021-068714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Xu J, Bai Y, Xu N, Li E, Wang B, Wang J, et al. Tislelizumab plus chemotherapy as first-line treatment for advanced esophageal squamous cell carcinoma and Gastric/Gastroesophageal junction adenocarcinoma. Clin Cancer Res an Off J Am Assoc Cancer Res (2020) 26(17):4542–50. doi: 10.1158/1078-0432.CCR-19-3561 [DOI] [PubMed] [Google Scholar]
- 181. Kato K, Cho BC, Takahashi M, Okada M, Lin CY, Chin K, et al. Nivolumab versus chemotherapy in patients with advanced oesophageal squamous cell carcinoma refractory or intolerant to previous chemotherapy (ATTRACTION-3): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol (2019) 20(11):1506–17. doi: 10.1016/S1470-2045(19)30626-6 [DOI] [PubMed] [Google Scholar]
- 182. Huang J, Xu J, Chen Y, Zhuang W, Zhang Y, Chen Z, et al. Camrelizumab versus investigator's choice of chemotherapy as second-line therapy for advanced or metastatic oesophageal squamous cell carcinoma (ESCORT): a multicentre, randomised, open-label, phase 3 study. Lancet Oncol (2020) 21(6):832–42. doi: 10.1016/S1470-2045(20)30110-8 [DOI] [PubMed] [Google Scholar]
- 183. Shen L, Kato K, Kim SB, Ajani JA, Zhao K, He Z, et al. Tislelizumab versus chemotherapy as second-line treatment for advanced or metastatic esophageal squamous cell carcinoma (RATIONALE-302): A randomized phase III study. J Clin Oncol Off J Am Soc Clin Oncol (2022) 40(26):3065–76. doi: 10.1200/JCO.21.01926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Doki Y, Ueno M, Hsu CH, Oh DY, Park K, Yamamoto N, et al. Tolerability and efficacy of durvalumab, either as monotherapy or in combination with tremelimumab, in patients from Asia with advanced biliary tract, esophageal, or head-and-neck cancer. Cancer Med (2022) 11(13):2550–60. doi: 10.1002/cam4.4593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Doi T, Piha-Paul SA, Jalal SI, Saraf S, Lunceford J, Koshiji M, et al. Safety and antitumor activity of the anti-programmed death-1 antibody pembrolizumab in patients with advanced esophageal carcinoma. J Clin Oncol Off J Am Soc Clin Oncol (2018) 36(1):61–7. doi: 10.1200/JCO.2017.74.9846 [DOI] [PubMed] [Google Scholar]
- 186. Shah MA, Kojima T, Hochhauser D, Enzinger P, Raimbourg J, Hollebecque A, et al. Efficacy and safety of pembrolizumab for heavily pretreated patients with advanced, metastatic adenocarcinoma or squamous cell carcinoma of the esophagus: The phase 2 KEYNOTE-180 study. JAMA Oncol (2019) 5(4):546–50. doi: 10.1001/jamaoncol.2018.5441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Li C, Zhao S, Zheng Y, Han Y, Chen X, Cheng Z, et al. Preoperative pembrolizumab combined with chemoradiotherapy for oesophageal squamous cell carcinoma (PALACE-1). Eur J Cancer (Oxford Engl 1990). (2021) 144:232–41. doi: 10.1016/j.ejca.2020.11.039 [DOI] [PubMed] [Google Scholar]
- 188. Kelly RJ, Ajani JA, Kuzdzal J, Zander T, Van Cutsem E, Piessen G, et al. Adjuvant nivolumab in resected esophageal or gastroesophageal junction cancer. New Engl J Med (2021) 384(13):1191–203. doi: 10.1056/NEJMoa2032125 [DOI] [PubMed] [Google Scholar]
- 189. Kudo T, Hamamoto Y, Kato K, Ura T, Kojima T, Tsushima T, et al. Nivolumab treatment for oesophageal squamous-cell carcinoma: an open-label, multicentre, phase 2 trial. Lancet Oncol (2017) 18(5):631–9. doi: 10.1016/S1470-2045(17)30181-X [DOI] [PubMed] [Google Scholar]
- 190. Zang YS, Dai C, Xu X, Cai X, Wang G, Wei J, et al. Comprehensive analysis of potential immunotherapy genomic biomarkers in 1000 Chinese patients with cancer. Cancer Med (2019) 8(10):4699–708. doi: 10.1002/cam4.2381 [DOI] [PMC free article] [PubMed] [Google Scholar]