Abstract
Background:
A high density of CD8+ tumor infiltrating lymphocytes (TILs) is associated with improved survival in multiple cancers, but its prognostic role in prostate cancer remains controversial. The aim of our study was to evaluate the prognostic value of CD8+ TILs in prostate cancer patients undergoing radical prostatectomy (RP). We hypothesized that elevated density of CD8+ TILs in the RP specimen would correlate with improved clinical outcomes. This information may be helpful for future immunotherapy clinical trial design and treatment selection.
Methods:
Tumor microarrays constructed from 230 patients with localized prostate cancers who underwent RP from 2006 to 2012 at Roswell Park Comprehensive Cancer Center were analyzed retrospectively using immunohistochemistry. CD8+ cell density was evaluated using a computerized scoring system. The cohorts were separated by CD8+ TIL density at the 25th percentile (i.e. low < quartile 1 and high ≥ quartile 1). The quartile 1 threshold was chosen through a “minimal P-value approach” based on overall survival with correction of significance to adjust for multiple testing. Clinical outcomes were compared in the high versus low CD8+ TIL density groups.
Results:
149 (65%) patients had high risk diseases (Gleason>7 or pT3/4). The median follow-up time was 8.4 years. High CD8+ TIL density was associated with improved 5-year overall survival (98% vs 91%, p = 0.01) and prostate cancer-specific survival (99% vs 95%, p = 0.04) compared to patients with low CD8+ TIL density. There was a trend toward higher 5-year biochemical recurrence-free survival and metastasis-free survival in the cohort of patients with high CD8+ TIL density (52% vs 38% and 86% vs 73%, respectively), although the difference did not reach statistical significance (p = 0.18 and p = 0.05, respectively). In a multivariate analysis high CD8+ TIL density was an independent favorable prognostic factor for overall survival (HR = 0.38, CI 95% 0.17–0.87, p = 0.02). In contrast to the prognostic value of CD8+ TIL density, the CD8+ cell density in the matched normal prostate tissue was not associated with any clinical outcomes.
Conclusions:
Intratumoral CD8+ T-cell infiltration in the RP specimen is independently associated with improved survival after RP in this high-risk prostate cancer cohort. Pre-RP immunomodulation that promotes intratumoral CD8+ cytotoxic T-cell infiltration may be beneficial for this population.
Introduction
Prostate cancer (PCa) is the most common cancer in men globally.1 In 2015, there were approximately 1.6 million incident cases of prostate cancer and 366,000 associated deaths. The current standard of care is quite effective in curing low to intermediate risk patients, but the management of high risk patients remains challenging. The majority of high risk patients will experience disease recurrence.2 More than half of these cases recur as distant metastasis.3 No available treatment is curative in the metastatic setting. Therefore, durable therapeutic strategies are necessary for high risk patients. Immune check-point inhibitors (ICI), especially the blockers of the Programmed Cell Death-1 (PD-1) axis, which counteract the functional suppression of CD8+ cytotoxic T cells (CTLs), have produced some unprecedented long-lasting responses in cancer immunotherapy. ICI treatment is especially effective in “hot” tumors containing high numbers of CTLs and high levels of intratumoral PD-L1 expression.4 However, the efficacy of ICI in treating PCa has been disappointing.5 A better understanding of PCa tumor infiltrating lymphocytes (TILs) and their microenvironment may improve our ability to target PCa immunologically, especially in view of the explosion of “immune” targets and new tools to treat cancer.
Many reports provide evidence of the positive prognostic role of TILs in many cancers, 6–10 but the role of TILs in PCa and their composition remain controversial. The majority of studies show predominant infiltration by CD4+ T lymphocytes and only limited influx of CD8+ T cells.11 The tumor-associated CD4+ cells expressed high levels of regulatory T-cell (Treg) markers CD25 and FOXP3 and were associated with shorter biochemical recurrence-free survival (BRFS) and higher PCa-specific mortality.12–15 The prognostic significance of CD8+ TILs in PCa is uncertain. Ness et al. reported that high CD8+ TIL density was associated with shorter BRFS.16 In contrast, Sorrentino et al. found that high numbers of CD8+ TILs after neoadjuvant androgen deprivation therapy (ADT) was associated with improved overall survival (OS).17 Moreover, Davidson et al. showed that the number of CD8+ TILs had no association with lethal PCa.15 A large study of PCa immune contexture using estimated bioinformatical deconvolution and mRNA levels did not consider the prognostic value of CD8+ TILs—all T cells were lumped together and their abundance was associated with worse metastasis-free survival (MFS).18 In view of a paucity of data, we retrospectively studied the prognostic significance of CD8+ TIL density in 230 men who underwent RP.
Methods
Patients
A cohort of 290 patients with localized PCa who underwent RP at Roswell Park Comprehensive Cancer Center from 2006 to 2012 were studied. Clinical outcomes were collected from a prospectively-populated quality assurance database. OS was defined as the time from RP until death due to any cause or last follow-up. PCa-specific survival was defined as the time from RP until death due to PCa or last follow-up. BRFS was defined as the time from RP until prostate-specific antigen (PSA) recurrence or last clinic follow-up. Patients with PSA persistence were not included in BRFS calculations. Metastasis free survival (MFS) was defined as the time from RP until development of metastatic disease (defined as suspicious lesions on CT and/or bone scans which were obtained if clinically indicated) or last clinic follow-up. PSA persistence and recurrence were defined using National Comprehensive Cancer Network guidelines.19 PSA persistence after RP was defined as failure of PSA to fall to undetectable levels (PSA = 0.04 ng/mL at our center); PSA recurrence was defined as undetectable PSA after RP with a subsequent detectable PSA that increased on 2 or more subsequent determinations. RP-failure included PSA persistence, PSA recurrence and post-RP treatment (ADT or radiotherapy).
Tumor Microarray Construction
Tumor microarrays (TMAs) were prepared using standard procedures as described.20 Briefly, TMAs blocks were constructed using the Beecher tissue puncher and array system (Beecher Instruments, Silver Spring, MD). The core sites were chosen randomly in the tumor regions to avoid bias. Each core measured 0.6-mm in diameter. Six tumor and three non-tumor cores were included for each patient. Specimens for controls within the TMA consisted of multiple cores of normal tissue from 10 different organs including brain, breast, lung, spleen, kidney, liver, tonsil, colon, testes, and ovary thereby representing more than 20% of all the cores in a TMA. Appropriate Institutional Review Board approval consistent with federal, state and local requirements was obtained for this project and clinical and outcome data was de-identified.
Immunohistochemical Staining
TMA sections were added to the Dako Omnis autostainer, where they were deparaffinized with Clearify (American Mastertech; catalog #CACLEGAL) and rinsed in water. Flex TRS High (Dako; catalog #GV804) was used for target retrieval for 30 minutes. Sections were incubated with CD8 (Dako #M7103) for 10 minutes at 1/50. Mouse Linker (Dako #GV821) was applied for 10 minutes followed by horseradish peroxidase for 15 mins (Dako GV823). Diaminobenzidine (Dako; catalog #K3468) was applied for 5 minutes for visualization. Slides were counterstained with hematoxylin for 8 minutes then placed into water. Sections were dehydrated, cleared and coverslipped.
Slide Annotation and Scoring
TMA sections were scanned digitally using Aperio ScanScope (Aperio Technologies, Inc., Vista, CA) with 20x bright-field microscopy. Images were accessed using Spectrum™ (Aperio Technologies, Inc., Vista, CA), a web-based digital pathology information management system. Section images were associated to a digital slide created in the Digital Slide table in Spectrum™.
Aperio ImageScope version 11.2.0.780 (Aperio) was used to view images for image analysis. Slide image data fields were populated, images were examined for quality and were amended as necessary. An annotation layer was created for each TMA core of interest. Each core was circled as area for analysis using the free form pen tool. Areas to be excluded, such as folded or necrotic tissue, were marked using the negative free form pen to remove these regions from image analysis calculations.
The Aperio™ platform was used to develop quantitative image analysis algorithm macros for quantification of immunostaining. Briefly, these algorithms used color de‐convolution to separate diaminobenzidine from the hematoxylin counterstain. A cytoplasmic algorithm was tailored to distinguish lymphocytes using cellular, nuclear, and immunostain parameters to produce an algorithm macro based on the cell compartment location of the target protein. An analysis macro was developed using a cytoplasmic algorithm from Aperio due to the small size of the CD8+ T cells. The results provided the total number of CD8+ T cells and the area of analysis. The number of CD8+ T lymphocytes was reported per square millimeter using a simple conversion formula in Excel.
Statistical Analysis
Patients with >5-year follow-up or RP-failure were included in the statistical analysis. The primary clinical outcome was OS. The average CD8+ cell density was calculated separately from PCa and non-tumor cores for each patient. The CD8+ cell density was dichotomized at the 25th percentile (i.e. low <quartile 1 and high ≥quartile 1). The quartile 1 cutoff was determined using the “minimal P-value approach” based on OS.21 To avoid potential bias from the maximization approach used to generate the CD8+ TIL density threshold, methods similar to Contal and O’Quigley were used to correct the log-rank p-value of the OS analysis.
Patient characteristics were reported using the median and range for continuous variables, and frequencies and relative frequencies for categorical variables. Comparisons were made between tumor TIL density groups (low versus high) using the Mann-Whitney U and chi-square tests for continuous and categorical variables, respectively. The time-to-event outcomes (OS, PCa specific survival, BRFS and MFS) were summarized using standard Kaplan-Meier methods, where estimates of the median and 5-year rates were obtained with 95% confidence intervals. Comparisons were made using the log-rank test. Cox regression models were used to evaluate the association between the time-to-event outcomes and CD8+ TIL density while adjusting for pre-RP PSA, pathological Gleason grade, and pathological T-stage. The models were fit using Firth’s method and hazard ratios, with corresponding 95% confidence intervals, were obtained from model estimates. All analyses were completed in SAS v9.4 (Cary, NC) at a significance level of 0.05.
Results
Patient Demographics and Clinicopathological Characteristics
230 of 290 patients with sufficient follow-up data were included in the statistical analysis. The median follow-up time was 100.3 months (range 0.3–137.7 months). A total of 149 patients (65%) were high risk—defined as Gleason score (GS)>7 or pT3/4. No patients received neoadjuvant treatment. Patient demographics, clinical and histopathological characteristics by CD8+ TIL density, and cancer recurrent status are presented in Table 1 and supplementary Table 1, respectively. There were no significant differences in baseline characteristics compared between the CD8+ TIL density-high and -low group.
Table 1.
Patient Characteristics and Clinicopathological Variables by CD8+ TIL Density
| CD8 TIL density | Low (N=57) | High (N=173) | P-value | |
|---|---|---|---|---|
| Age | Median (Range) | 59 (44–73) | 61 (40–79) | 0.51 |
| Pre-RP PSA | < 10 | 39 (69 %) | 138 (80 %) | 0.13 |
| 10–20 | 11 (19 %) | 17 (10 %) | ||
| >= 20 | 7 (12 %) | 18 (10 %) | ||
| Path Gleason Sum | < 7 | 10 (18 %) | 32 (18 %) | 0.72 |
| = 7 | 28 (49 %) | 93 (54 %) | ||
| > 7 | 19 (33 %) | 48 (28 %) | ||
| Path T Stage | pT2 | 17 (30 %) | 75 (43 %) | 0.15 |
| pT3 | 32 (56 %) | 83 (48 %) | ||
| pT4 | 8 (14 %) | 15 (9 %) | ||
| Path N Status | Positive | 2 (4 %) | 12 (7 %) | 0.45 |
| Margin Status | Positive | 25 (44 %) | 65 (38 %) | 0.40 |
| Adjuvant ADT | Yes | 1 (2 %) | 3 (2 %) | 0.99 |
| Adjuvant RT | Yes | 8 (14 %) | 20 (12 %) | 0.62 |
| Post-RP Risk Group | Low-Intermediate | 15 (26%) | 66 (38%) | 0.10 |
| High | 42 (74%) | 107 (62%) | ||
Note. RP = radical prostatectomy; ADT = androgen deprivation therapy; RT = radiotherapy; Path= pathological; TIL = tumor infiltrating lymphocyte; T = tumor; N = lymph node; Post-RP Low-Intermediate Risk = path Gleason <= 7 and pT2; Post-RP High Risk = path Gleason > 7 or pT3/4
Evaluation of CD8+ TIL density by Immunohistochemistry
Representative images of tissue sections are shown in Figure 1. The median CD8+ TIL density of the entire cohort was 51 cells/mm2 (interquartile range 30–87). To study the relationship between CD8+ TIL density and clinical outcomes, patients were divided into high and low density groups. The minimal p-value approach was used to identify an optimal cutoff value of the continuous variable based on OS.21 The value obtained from this approach was approximately the value of quartile 1, which was used to differentiate low (N=57, 25%) and high (N=173, 75%) CD8+ TIL density. The median cell density of the low group was 21 cells/mm2 (interquartile range 15–26) compared to 62 cells/mm2 in the high group (interquartile range 46–101).
Figure 1. CD8 immunohistochemical staining of primary prostate cancer tissue microarrays.
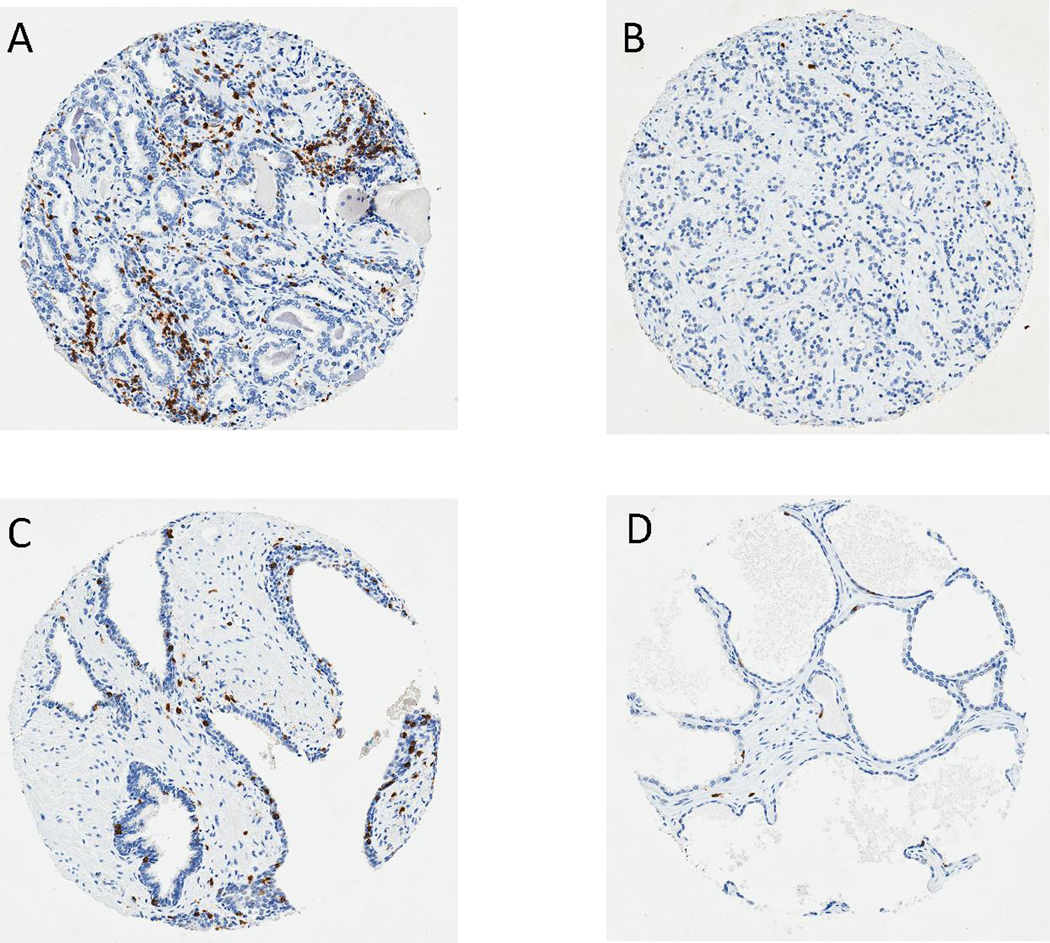
Representative images (100 X) of CD8 immunostaining (in brown) show (A) Tumor core with high CD8+ TIL density; (B) Tumor core with low CD8+ TIL density; (C) Benign core with high CD8+ TIL density; (D) Benign core with low CD8+ TIL density.
Associations between Time-to-Event Outcomes and CD8+ TIL density
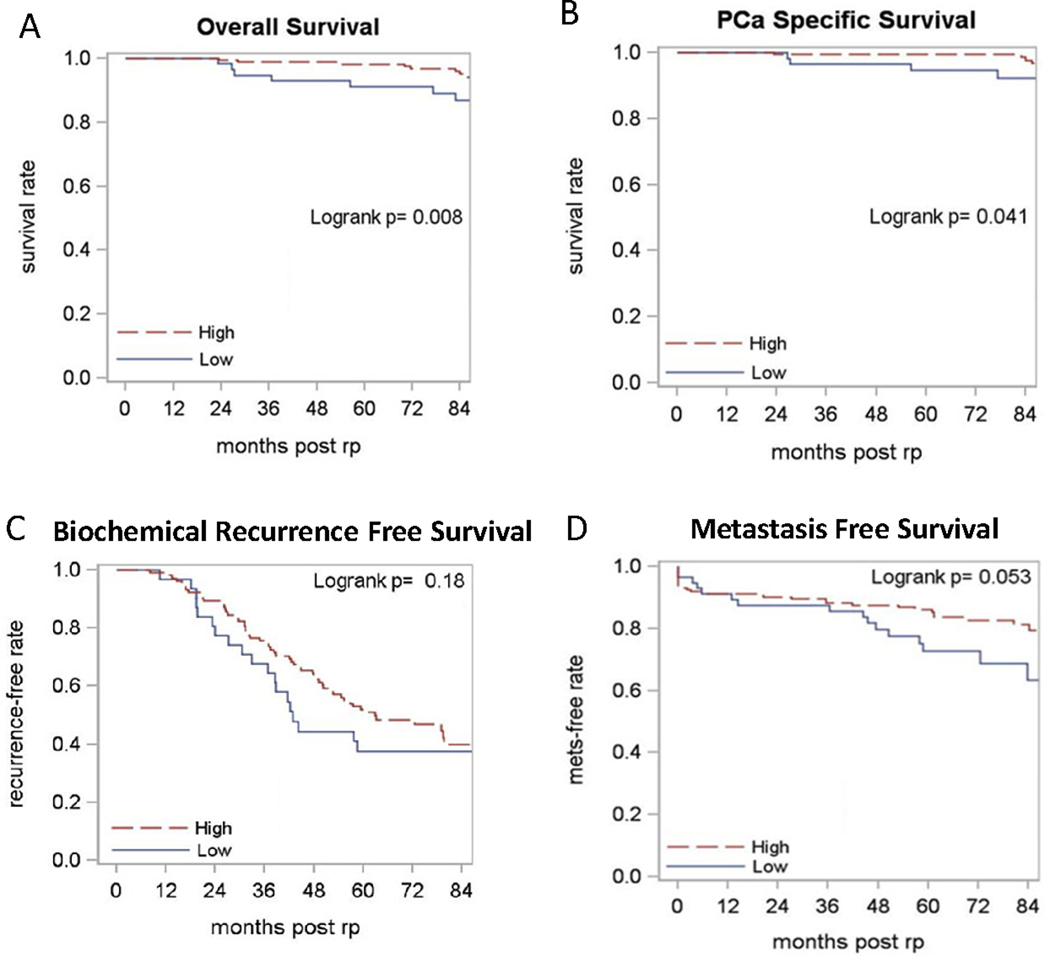
High CD8+ TIL density was associated with improved 5-year OS (98% vs 91%, p = 0.01) and PCa-specific survival (99% vs 95%, p = 0.04) compared to the low CD8+ TIL density group (Figure 2A/B). The 5-year BRFS and MFS were higher in the high CD8+ TIL density group (52% vs 38% and 86% vs 73% respectively), but the difference did not reach statistical significance (p = 0.18 and p = 0.05 respectively, Figure 2C/D). In contrast, no significant associations were observed between CD8+ T-cell densities in patients’ normal tissues and any of the clinical outcomes evaluated (data not shown).
Figure 2. The Kaplan-Meier curves of groups with high vs low CD8+ TIL density.
Red lines represent high CD8+ TIL density whereas blue lines indicate low CD8+ TIL density. The curves are compared using Log Rank Analysis. (A) Overall survival; (B) Prostate cancer-specific survival; (C) Biochemical recurrence-free survival (N=137); (D) Metastasis-free survival.
Subgroup analysis by post-RP risk group
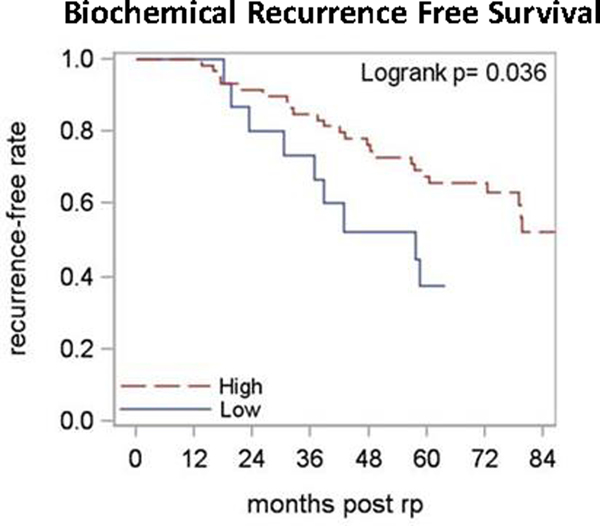
CD8+ TIL density and post-RP risk group were confirmed as independent variables by 2×2 Chi-square analysis (P-value by Fisher’s exact test = 0.10). Among the low-intermediate risk patients (GS≤7 & pT2; N = 81), high CD8+ TIL density was associated with improved 5-year BRFS (68% vs 38%, p = 0.04, Figure 3). The number of events was too few to analyze for OS, PCa specific survival and MFS differences.
Figure 3. The Kaplan-Meier curves of patients with low-intermediate risk prostate cancer.
Low-intermediate risk after radical prostatectomy is defined as path GS<=7 and pT2. Red lines represent high CD8+ TIL density whereas blue lines indicate low CD8+ TIL density. The biochemical recurrence-free survival curves are compared using Log Rank Analysis (N=75).
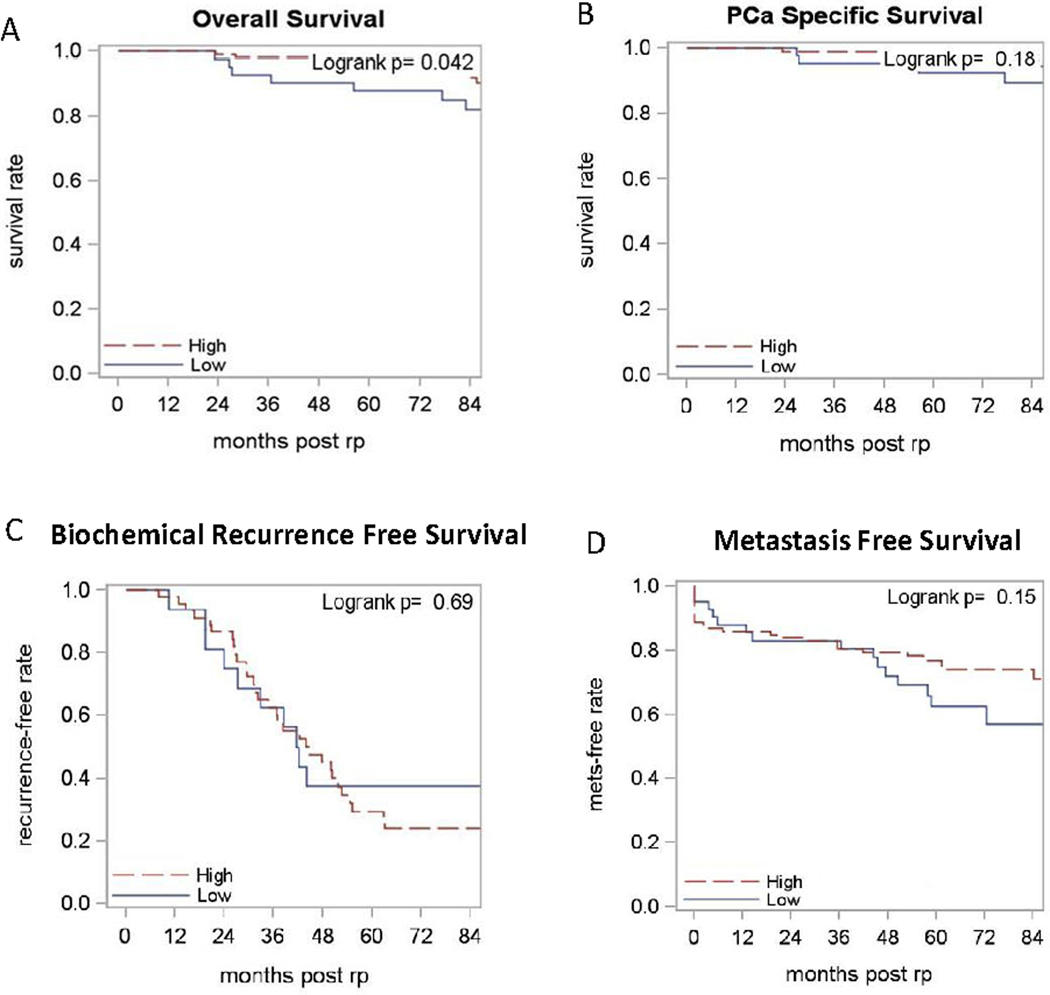
Within the high risk sub-group (GS≥8 or pT3/4; N = 149), a statistically significant association was observed for CD8+ TIL density and OS (p = 0.04); patients with low CD8+ TIL density had inferior 5-year OS (88% vs 97%, Figure 3A). The 5-year PCa-specific survival and MFS were numerically better in patients with high CD8+ TILs (99% vs 93% and 77% vs 63% respectively, Figure 4B/D). However, statistical significance was not reached (p = 0.18 and p = 0.15, respectively). The BRFS of high risk patients was similar (p = 0.69), regardless of the CD8+ TIL density (Figure 4C).
Figure 4. The Kaplan-Meier curves of patients with high risk prostate cancer.
High risk after radical prostatectomy is defined as path GS>7 or pT3/4 (N=149). Red lines represent high CD8+ TIL density whereas blue lines indicate low CD8+ TIL density. The curves are compared using Log Rank Analysis. (A) Overall survival; (B) Prostate cancer-specific survival; (C) Biochemical recurrence-free survival (N=62); (D) Metastasis-free survival.
Multivariate Survival Analysis
Time-to-event outcomes were modeled as a function of CD8+ TIL density, pathological Gleason grade, pathological T-stage and pre-RP PSA. High CD8+ TIL density was independently associated with OS (HR = 0.38, CI 95% 0.17–0.87, p = 0.02). The associations of PCa-specific survival, BRFS and MFS with high CD8+ TIL density were not statistically significant (HR=0.30, 0.60, 0.63; p = 0.08, p = 0.08 and p = 0.14, respectively). Pathological Gleason grade appeared to be a factor associated with BRFS and MFS (p <0.01 and p = 0.02, respectively), while pathological T-stage appeared to be associated with all outcomes except PCa-specific survival (p = 0.39). Pre-op PSA did not prove to be an independent predictor of the outcomes. The full results are shown in Table 2.
Table 2.
Multivariable Analysis of Time-to-Event Outcomes
| Clinical Outcome | Variable | Hazard Ratio (95% CI) | P-value | |
|---|---|---|---|---|
| Overall Survival | TIL density | High vs Low | 0.38 (0.17, 0.87) | 0.02 |
| Path Gleason Sum | >7 vs <7 =7 vs <7 |
2.19 (0.33, 14.54) 1.34 (0.21, 8.45) |
0.48 | |
| Path T-Stage | pT4 vs pT2 pT3 vs pT2 |
2.19 (0.33, 14.80) 6.00 (1.41, 25.57) |
0.03 | |
| Pre-RP PSA | >=20 vs <10 10–20 vs <10 |
1.79 (0.58, 5.51) 1.61 (0.53, 4.92) |
0.49 | |
| PCa-Specific Survival | TIL density | High vs Low | 0.30 (0.08, 1.14) | 0.08 |
| Path Gleason Sum | >7 vs <7 =7 vs <7 |
3.74 (0.12, 112.58) 2.56 (0.09, 70.83) |
0.71 | |
| Path T-Stage | pT4 vs pT2 pT3 vs pT2 |
0.63 (0.02, 22.00) 2.87 (0.40, 20.60) |
0.39 | |
| Pre-RP PSA | >=20 vs <10 10–20 vs <10 |
1.36 (0.19, 9.83) 1.88 (0.36, 9.87) |
0.75 | |
| Biochemical Recurrence-Free Survival | TIL density | High vs Low | 0.60 (0.35, 1.05) | 0.08 |
| Path Gleason Sum | >7 vs <7 =7 vs <7 |
1.42 (0.54, 3.70) 4.89 (2.28, 10.51) |
<.01 | |
| Path T-Stage | pT4 vs pT2 pT3 vs pT2 |
2.20 (0.80, 6.03) 2.85 (1.67, 4.84) |
<.01 | |
| Pre-RP PSA | >=20 vs <10 10–20 vs <10 |
1.40 (0.54, 3.62) 1.03 (0.44, 2.41) |
0.79 | |
| Metastasis-Free Survival | TIL density | High vs Low | 0.63 (0.35, 1.15) | 0.14 |
| Path Gleason Sum | >7 vs <7 =7 vs <7 |
22.64 (1.26, 406.88) 12.40 (0.70, 220.05) |
0.02 | |
| Path T-Stage | pT4 vs pT2 pT3 vs pT2 |
3.04 (1.08, 8.56) 2.59 (1.12, 6.00) |
0.06 | |
| Pre-RP PSA | >=20 vs <10 10–20 vs <10 |
1.67 (0.78, 3.58) 1.21 (0.53, 2.77) |
0.41 |
Note. RP = radical prostatectomy; ADT = androgen deprivation therapy; RT = radiotherapy; Path = pathological; TIL = CD8+ tumor infiltrating lymphocyte; T = tumor; N = lymph node
Discussion
Data from the retrospective analysis indicates that high CD8+ TIL density in RP specimens is independently associated with favorable OS in patients with localized PCa, and the prognostic value remains significant after adjustment for other clinicopathological factors. In contrast, the CD8+ T-lymphocyte density of the adjacent normal prostate tissues had no prognostic value. The improved outcome appeared to be driven by the local immune response within the tumor.
In addition to the primary clinical outcome of OS, several secondary outcomes were included to test internal consistency. In agreement with the OS analysis, high CD8+ TIL density was associated with improved PCa-specific survival. However, the BRFS and MFS differences, though trending in the same direction, did not reach a level of significance (p = 0.18 and 0.05 respectively). These discrepancies are likely due to the limitations of retrospective analysis. For example, radiographic studies are not routinely performed as standard of care, the detection of asymptomatic metastasis may be delayed and BRFS were underpowered [e.g., 89 (39%) patients had PSA persistence who were excluded from BRFS analysis]. Among the high risk patients, there were differences in OS but not BRFS. This may be explained by the poor correlation of BRFS and OS in patients with localized PCa after RP.22
Our findings are in contrast to a similar study by Ness et al. who concluded that high CD8+ TIL density was associated with early biochemical failure, although no survival data was reported.16 The conflicting results may be explained by: 1) a larger proportion of high risk patients were included in our study, 2) we evaluated more cores per patient (6 vs 2 tumor cores) to take into account tumor heterogeneity and, 3) we adapted a computer-based automated scoring system to avoid operator-dependent variables. Nevertheless, our results agree with a report by Vicier et al. who showed that high CD8+ TIL density at RP was associated with improved BRFS and MFS.23 Taken together, low CD8+ TIL density at RP may prove a useful biomarker to identify, a priori, patients at high risk of disease progression and mortality.
High intratumoral PD-L1 expression may be another useful biomarker to predict poor prognosis at the time of RP.23,24 However, the efficacy of ICI targeting PD-1/PD-L1 axis remains disappointing in PCa.25–28 The presence of high numbers of TILs is a prerequisite for ICI response.29 In the current study, we observed a median CD8+ TIL density of 51 cells/mm2 in primary PCa. This is significantly lower than its melanoma counterpart, where the median CD8+ TIL density is about 2,500 cells/mm2 in ICI responders.30 Our data suggest that the “cold” immune tumor microenvironment of PCa is accounted for, at least in part, by quantitative deficiency of CTLs. ADT, sipuleucel-T and granulocyte-macrophage colony-stimulating factor can independently induce TIL influx into PCa.31–34 However, the CTL recruitment is often accompanied by a large amount of Treg cells, which dampen anti-tumor activity. Prostvac, a cancer vaccine, can enhance intratumoral T-cell infiltration, but in a CD4+ cell-predominant fashion.35 Ipilimumab, an anti-CTLA4 antibody, induces a pronounced compensatory upregulation of other ICI molecules, such as PD-L1 and VISTA.36 Therefore, co-inhibition of Treg or multiple ICIs, while promoting CTL influx, may be a future direction for PCa immunotherapy. A therapeutic opportunity is presented by our recent observations that the combination of Toll-like receptor 3 ligands and Interferon-α selectively enhances the production of CTL attractants but suppresses intratumoral production of Treg attractant CCL22 in PCa tumor explants ex vivo.37 Verification of the ability of this combination to manipulate the tumor microenvironment of PCa patients may provide for a novel tool to enhance CTL influx and sensitize “cold” PCa to ICI.
Our study has several limitations. TMA was chosen for its cost-effectiveness with large sample size analysis. Although IHC generally requires at least 3 cores per section to accurately represent the whole section, even 6 cores may not capture the heterogeneity of a bulky tumor.38 The TMA approach prevented evaluation of the transition between malignant and normal tissue, which has been recognized as a useful factor to differentiate immunophenotypes of individual tumors.39 Another limitation of this study is the use of a single marker for CD8. Since not every CD8+ cell is a cytotoxic T cell,40 our future multiplex IHC study will sub-classify CD8+ TILs using functional markers such as granzymes, FOXP3, and PD-1.
To our knowledge, this is the first study demonstrating that high CD8+ TIL density at the time of RP is independently associated with improved OS in men with localized high risk PCa. The majority of men with high risk PCa either have persistent disease or develop cancer relapse after RP. Neoadjuvant immunotherapeutic manipulations to promote CD8+ T cell influx into the PCa may confer a survival benefit. Furthermore, low CD8+ TIL density at the time of RP may also identify a group of patients with a higher risk of mortality who may benefit from aggressive adjuvant therapy.
Supplementary Material
Acknowledgements
This work was supported by National Cancer Institute (NCI) grant P30CA016056, and S10OD019977 involving the use of Roswell Park Comprehensive Cancer Center’s Biostatistics & Statistical Genomics and Pathology Network Shared Resource.
Footnotes
Disclosure/Conflict of Interest
None.
References
- 1.Global Burden of Disease Cancer C, Fitzmaurice C, Allen C, et al. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-years for 32 Cancer Groups, 1990 to 2015: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol 2017;3:524–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pierorazio PM, Ross AE, Lin BM, et al. Preoperative characteristics of high-Gleason disease predictive of favourable pathological and clinical outcomes at radical prostatectomy. BJU Int 2012;110:1122–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marzola MC, Chondrogiannis S, Ferretti A, et al. Role of 18F-choline PET/CT in biochemically relapsed prostate cancer after radical prostatectomy: correlation with trigger PSA, PSA velocity, PSA doubling time, and metastatic distribution. Clin Nucl Med 2013;38:e26–32. [DOI] [PubMed] [Google Scholar]
- 4.Gajewski TF, Schreiber H, Fu Y-X. Innate and adaptive immune cells in the tumor microenvironment. Nature Immunology 2013;14:1014–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Redman JM, Gulley JL, Madan RA. Combining immunotherapies for the treatment of prostate cancer. Urol Oncol 2017;35:694–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ropponen KM, Eskelinen MJ, Lipponen PK, Alhava E, Kosma VM. Prognostic value of tumour-infiltrating lymphocytes (TILs) in colorectal cancer. The Journal of pathology 1997;182:318–24. [DOI] [PubMed] [Google Scholar]
- 7.Naito Y, Saito K, Shiiba K, et al. CD8+ T cells infiltrated within cancer cell nests as a prognostic factor in human colorectal cancer. Cancer Res 1998;58:3491–4. [PubMed] [Google Scholar]
- 8.Pages F, Berger A, Camus M, et al. Effector memory T cells, early metastasis, and survival in colorectal cancer. N Engl J Med 2005;353:2654–66. [DOI] [PubMed] [Google Scholar]
- 9.Galon J, Costes A, Sanchez-Cabo F, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science 2006;313:1960–4. [DOI] [PubMed] [Google Scholar]
- 10.Gooden MJM, De Bock GH, Leffers N, Daemen T, Nijman HW. The prognostic influence of tumour-infiltrating lymphocytes in cancer: a systematic review with meta-analysis. British Journal of Cancer 2011;105:93–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ebelt K, Babaryka G, Figel AM, et al. Dominance of CD4+ lymphocytic infiltrates with disturbed effector cell characteristics in the tumor microenvironment of prostate carcinoma. Prostate 2008;68:1–10. [DOI] [PubMed] [Google Scholar]
- 12.Sfanos KS, Bruno TC, Maris CH, et al. Phenotypic analysis of prostate-infiltrating lymphocytes reveals TH17 and Treg skewing. Clin Cancer Res 2008;14:3254–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miller AM, Lundberg K, Ozenci V, et al. CD4+CD25high T cells are enriched in the tumor and peripheral blood of prostate cancer patients. J Immunol 2006;177:7398–405. [DOI] [PubMed] [Google Scholar]
- 14.Flammiger A, Weisbach L, Huland H, et al. High tissue density of FOXP3+ T cells is associated with clinical outcome in prostate cancer. Eur J Cancer 2013;49:1273–9. [DOI] [PubMed] [Google Scholar]
- 15.Davidsson S, Ohlson AL, Andersson SO, et al. CD4 helper T cells, CD8 cytotoxic T cells, and FOXP3(+) regulatory T cells with respect to lethal prostate cancer. Mod Pathol 2013;26:448–55. [DOI] [PubMed] [Google Scholar]
- 16.Ness N, Andersen S, Valkov A, et al. Infiltration of CD8+ lymphocytes is an independent prognostic factor of biochemical failure-free survival in prostate cancer. Prostate 2014;74:1452–61. [DOI] [PubMed] [Google Scholar]
- 17.Sorrentino C, Musiani P, Pompa P, Cipollone G, Di Carlo E. Androgen deprivation boosts prostatic infiltration of cytotoxic and regulatory T lymphocytes and has no effect on disease-free survival in prostate cancer patients. Clin Cancer Res 2011;17:1571–81. [DOI] [PubMed] [Google Scholar]
- 18.Zhao SG, Lehrer J, Chang SL, et al. The Immune Landscape of Prostate Cancer and Nomination of PD-L2 as a Potential Therapeutic Target. JNCI: Journal of the National Cancer Institute 2019;111:301–10. [DOI] [PubMed] [Google Scholar]
- 19.Mohler JL, Antonarakis ES, Armstrong AJ, et al. Prostate Cancer, Version 2.2019, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2019;17:479–505. [DOI] [PubMed] [Google Scholar]
- 20.Mhawech-Fauceglia P, Herrmann FR, Bshara W, et al. Friend leukaemia integration-1 expression in malignant and benign tumours: a multiple tumour tissue microarray analysis using polyclonal antibody. J Clin Pathol 2007;60:694–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Contal C, O’Quigley J. An application of changepoint methods in studying the effect of age on survival in breast cancer. Computational Statistics & Data Analysis 1999;30:253–70. [Google Scholar]
- 22.Jhaveri FM, Zippe CD, Klein EA, Kupelian PA. Biochemical failure does not predict overall survival after radical prostatectomy for localized prostate cancer: 10-year results. Urology 1999;54:884–90. [DOI] [PubMed] [Google Scholar]
- 23.Vicier C, Werner L, Huang Y, et al. Immune infiltrate with CD8 low or PDL1 high associated with metastatic prostate cancer after radical prostatectomy (RP). Journal of Clinical Oncology 2019;37:86-.30407898 [Google Scholar]
- 24.Petitprez F, Fossati N, Vano Y, et al. PD-L1 Expression and CD8+ T-cell Infiltrate are Associated with Clinical Progression in Patients with Node-positive Prostate Cancer. European Urology Focus 2019;5:192–6. [DOI] [PubMed] [Google Scholar]
- 25.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 2012;366:2443–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kwon ED, Drake CG, Scher HI, et al. Ipilimumab versus placebo after radiotherapy in patients with metastatic castration-resistant prostate cancer that had progressed after docetaxel chemotherapy (CA184–043): a multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol 2014;15:700–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beer TM, Kwon ED, Drake CG, et al. Randomized, Double-Blind, Phase III Trial of Ipilimumab Versus Placebo in Asymptomatic or Minimally Symptomatic Patients With Metastatic Chemotherapy-Naive Castration-Resistant Prostate Cancer. J Clin Oncol 2017;35:40–7. [DOI] [PubMed] [Google Scholar]
- 28.Antonarakis ES, Piulats JM, Gross-Goupil M, et al. Pembrolizumab for Treatment-Refractory Metastatic Castration-Resistant Prostate Cancer: Multicohort, Open-Label Phase II KEYNOTE-199 Study. Journal of Clinical Oncology 2020;38:395–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tang H, Wang Y, Chlewicki LK, et al. Facilitating T Cell Infiltration in Tumor Microenvironment Overcomes Resistance to PD-L1 Blockade. Cancer Cell 2016;29:285–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tumeh PC, Harview CL, Yearley JH, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 2014;515:568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mercader M, Bodner BK, Moser MT, et al. T cell infiltration of the prostate induced by androgen withdrawal in patients with prostate cancer. Proc Natl Acad Sci U S A 2001;98:14565–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gannon PO, Poisson AO, Delvoye N, Lapointe R, Mes-Masson AM, Saad F. Characterization of the intra-prostatic immune cell infiltration in androgen-deprived prostate cancer patients. J Immunol Methods 2009;348:9–17. [DOI] [PubMed] [Google Scholar]
- 33.Fong L, Carroll P, Weinberg V, et al. Activated lymphocyte recruitment into the tumor microenvironment following preoperative sipuleucel-T for localized prostate cancer. J Natl Cancer Inst 2014;106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wei XX, Chan S, Kwek S, et al. Systemic GM-CSF Recruits Effector T Cells into the Tumor Microenvironment in Localized Prostate Cancer. Cancer Immunol Res 2016;4:948–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Abdul Sater H, Marté JL, Donahue RN, et al. Neoadjuvant PROSTVAC prior to radical prostatectomy enhances T-cell infiltration into the tumor immune microenvironment in men with prostate cancer. Journal for ImmunoTherapy of Cancer 2020;8:e000655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gao J, Ward JF, Pettaway CA, et al. VISTA is an inhibitory immune checkpoint that is increased after ipilimumab therapy in patients with prostate cancer. Nature Medicine 2017;23:551–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Muthuswamy R, Corman JM, Dahl K, Chatta GS, Kalinski P. Functional reprogramming of human prostate cancer to promote local attraction of effector CD8(+) T cells. Prostate 2016;76:1095–105. [DOI] [PubMed] [Google Scholar]
- 38.Scognamiglio G, Cantile M, Scala S, et al. Tissue micro arrays for immunohistochemical detection of inflammatory infiltrates in renal cell carcinoma. Int J Clin Exp Med 2014;7:1175–9. [PMC free article] [PubMed] [Google Scholar]
- 39.Joyce JA, Fearon DT. T cell exclusion, immune privilege, and the tumor microenvironment. Science 2015;348:74–80. [DOI] [PubMed] [Google Scholar]
- 40.Kiniwa Y, Miyahara Y, Wang HY, et al. CD8+ Foxp3+ regulatory T cells mediate immunosuppression in prostate cancer. Clin Cancer Res 2007;13:6947–58. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.