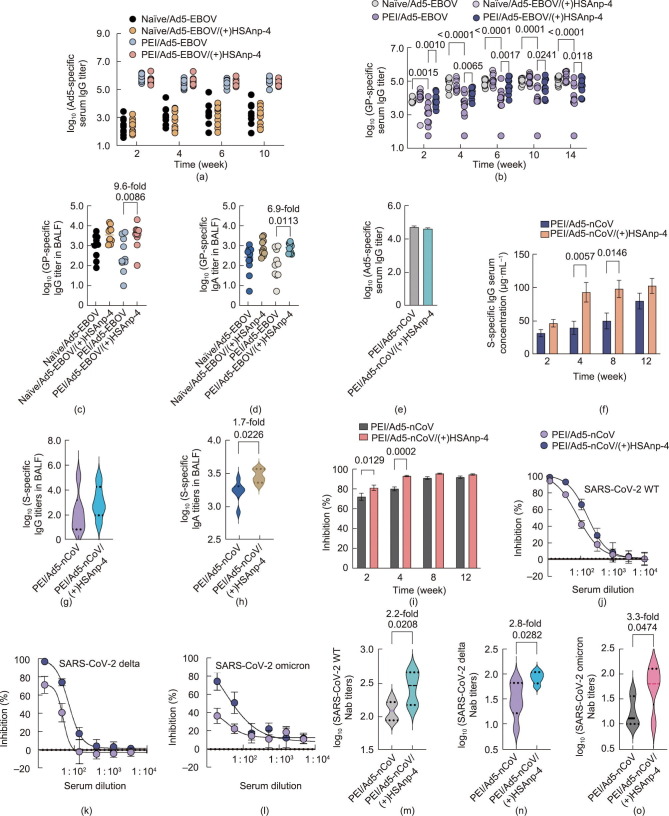
Fig. 5.
Complexed Ad5-based Ebola and COVID-19 vaccines overcome PEI. (a–d) BALB/c mice (n = 10) were immunized intranasally with a single dose of 5 × 106 IFU of Ad5-EBOV or Ad5-EBOV complexed with 1 μg (+)HSAnp-4 (1:1.2 × 104 molar ratio) in the absence (naïve) or presence of PEI. (a) Ad5-specific and (b) GP-specific serum IgG titers were measured at specific time points post vaccination. BALF was collected at week 14 post vaccination and assessed for GP-specific (c) IgG and (d) IgA titers. (e–m) In the presence of PEI, BALB/c mice were immunized intranasally with a single dose of 5 × 106 IFU of Ad5-nCoV or Ad5-nCoV complexed with 1 μg (+)HSAnp-4 (1:1.2 × 104 molar ratio). (e) Ad5-specific serum IgG titers were measured before vaccination. (f) SARS-CoV-2 S-specific IgG serum concentrations were assessed at 2, 4, 8, and 12 weeks post vaccination. BALF was collected at week 12 post vaccination and assessed for (g) S-specific IgG and (h) IgA titers. (i) A biochemical assay was used to measure the serum inhibition of RBD-hACE2 interactions. (j–o) Serum neutralizing antibody titers against (j, m) SARS-CoV-2 WT, (k, n) delta variant, and (l, o) omicron variant were measured by means of pseudovirus neutralization assays at 4 weeks post vaccination. Data are presented as means ± SEM. Statistical differences in parts (b), (f), and (i) were determined using a two-way ANOVA with Šidák’s multiple comparison test. Statistical differences in (c) and (d) were determined using ANOVA with Dunn’s multiple comparison test. Statistical differences in parts (g), (h), (m), (n), and (o) were determined using a two-tailed unpaired t-test.