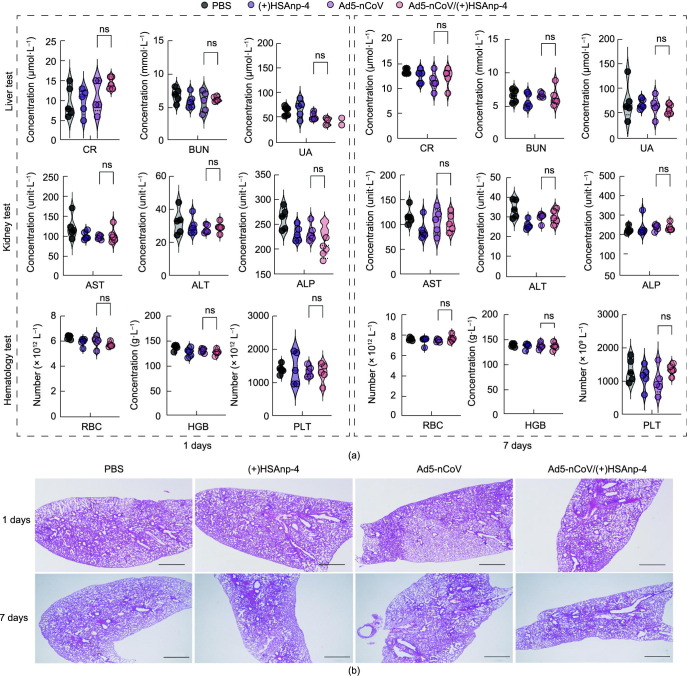
Fig. 6.
Evaluation of the safety profile of Ad5/(+)HSAnp. (a) BALB/c mice (n = 5) were intranasally administrated with a single dose of PBS, 1 μg of (+)HSAnp-4, 5 × 106 IFU of Ad5-nCoV, or 5 × 106 IFU of Ad5-nCoV complexed with 1 μg of (+)HSAnp-4 (1:1.2 × 104 molar ratio). (a) Blood tests and (b) histological analysis of lungs performed at 1 day and 7 days post administration (scale bar: 1000 μm). Data are presented as means ± SEM. Statistical differences were determined using ANOVA with Dunn’s multiple comparison test.