Fig. 7: Strategies for loading hexameric helicases.
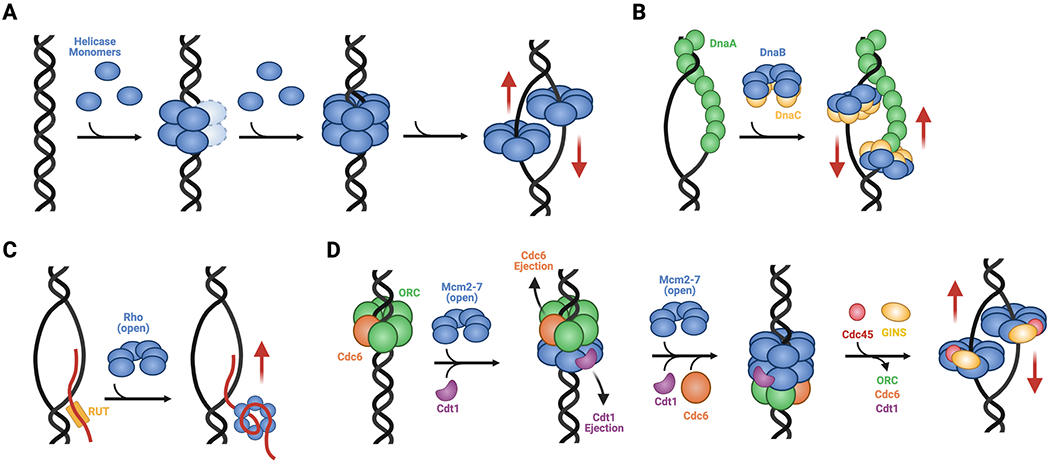
A. Ring assembly. SF-III helicases such as LTag and E1 are assembled at viral replication origins, proceeding through head-to-head intermediate complexes into single hexamers that encircle single DNA strands. B. Chaperoned rings opening. The SF-IV DnaB helicase from E. coli forms pre-assembled hexamers that are physically opened by DnaC and loaded onto a ssDNA bubble formed by the replication initiator, DnaA. C. Self-regulated ring closure. The SF-V helicase Rho forms a pre-opened hexamer that is recruited to target RNAs by Rho utilization (rut) sequences; once bound, the threading of ssRNA into the helicase channel promotes ring closure. D. Chaperoned ring closure. SF-VI helicases like MCMs form pre-opened hexameric rings that are loaded onto duplex replication origins by ORC, Cdc6, and Cdt1. This process establishes a closed-ring, head-to-head double hexamer intermediate that, when activated by Cdc45 and GINS to form the CMG complex, melts the origin and isomerizes into single hexamers that encircle ssDNA. Red arrows indicate direction of post-loading translocation. Helicase bypass following loading occurs for SF-III, -IV, and -VI enzymes.
