Abstract
Purpose:
Breast terminal duct lobular units (TDLUs) are the main source of breast cancer (BC) precursors. Higher serum concentrations of hormones and growth factors have been linked to increased TDLU numbers and elevated BC risk, with variable effects by menopausal status. We assessed associations of circulating factors with breast histology among premenopausal women using artificial intelligence (AI) and preliminarily tested whether parity modifies associations.
Methods:
Pathology AI analysis was performed on 316 digital images of H&E-stained sections of normal breast tissues from Komen Tissue Bank donors ages ≤45 years to assess 11 quantitative metrics. Associations of circulating factors with AI metrics were assessed using regression analyses, with inclusion of interaction terms to assess effect modification.
Results:
Higher prolactin levels were related to larger TDLU area (p<0.001) and increased presence of adipose tissue proximate to TDLUs (p<0.001), with less significant positive associations for acini counts (p=0.012), dilated acini (p=0.043), capillary area (p=0.014), epithelial area (p=0.007), and mononuclear cell counts (p=0.017). Testosterone levels were associated with increased TDLU counts (p<0.001), irrespective of parity, but associations differed by adipose tissue content. AI data for TDLU counts generally agreed with prior visual assessments.
Conclusion:
Among premenopausal women, serum hormone levels linked to BC risk were also associated with quantitative features of normal breast tissue. These relationships were suggestively modified by parity status and tissue composition. We conclude that the microanatomic features of normal breast tissue may represent a marker of BC risk.
Keywords: Breast Cancer Risk, Terminal Duct Lobular Units, Hormones, Normal Breast Tissue
Introduction
Breast terminal duct lobular units (TDLUs) are the site of milk production and the structures from which most breast cancer (BC) precursors develop [1]. Among young women, TDLU characteristics are strongly influenced by two involution processes: 1) postpartum involution, a relatively rapid process characterized by cell death, inflammation and wound healing, which occurs after weaning, and may increase BC risk [2–7] and 2) age-related lobular involution, a gradual process, which may begin as early as the fourth decade of life and is linked to lower BC risk [8–10]. Breast tissues of parous women contain greater numbers of TDLUs per unit area than nulliparas [10–12], but are comprised of epithelial cells that express greater degrees of differentiation based on RNA expression [13]. The sequential pattern of increase in numbers of lobules, postpartum involution and epithelial differentiation associated with the pregnancy-lactation cycle may be linked to the dualistic impact of childbirth on BC risk: transient increase, followed by later protection [13, 14]. Accordingly, we hypothesize that the association of circulating levels of hormones and growth factors with normal breast histology may vary between recently parous women, who have experienced postpartum involution, versus young nulliparous women, who have not. Given that delayed age-related TDLU involution in benign breast disease (BBD) biopsies has been associated with increased BC risk [8, 9], establishing associations of circulating marker concentrations and TDLU involution levels may suggest that the latter provide intermediate biomarkers of BC risk that reflect the integrated effects of numerous influences over time [10]. Improving risk prediction among young women is a priority, given that incidence rates of premenopausal BCs are rising and that delays in childbearing may magnify transient increases in BC risk after a birth [14].
Circulating hormones and growth factors are among the best studied markers of premenopausal BC risk. A pooled multivariate analysis of premenopausal women (767 BC cases diagnosed at ages ≤ 50 years and 1699 controls) found modest associations of increased circulating estrogens and androgens with increased BC risk of ≤ 30%, whereas luteal phase progesterone, sex-hormone binding globulin (SHBG) and calculated free testosterone concentrations were not significant predictors of overall BC risk [15]. However, luteal phase progesterone levels were associated with BC risk among nulliparas [15]. In the largest study to date conducted within the UK Biobank, Tin et al. found that among premenopausal women elevated IGF-1 was associated with minimally increased BC risk, but results for testosterone and SHBG were null, whereas among postmenopausal women, positive risk relationships were found for testosterone and IGF-1 (estradiol was not assessed) [16]. Prior pooled studies have found associations of increased estradiol and BC risk in selected analyses of premenopausal women [15] and consistently among postmenopausal women [17]. Studies also link prolactin levels to BC [18–20], but it is uncertain whether associations vary by menopausal status and whether prolactin is an etiological factor or a biomarker of undetected BC [20, 21]. Finally, a pooled analysis (4,790 BC cases and 9,428 controls) found that elevated IGF-1 was associated with higher premenopausal (and postmenopausal) BC risk, albeit modestly [22].
Defining the relationships of premenopausal hormone levels to TDLU involution and to BC risk is complex because of difficulties in accounting for the dramatic changes that occur during pregnancy, lactation, and the postpartum period [2–5, 7] and challenges related to accurately adjusting circulating hormone levels for menstrual dates. Specifically, menstrual dates at blood donation are projected from the self-reported date of the last menstrual period based on an idealized 28-day cycle. Given that irregular cycles are common among young women and that hormone levels fluctuate dramatically from day to day of the cycle (i.e., peri-ovulatory estrogen spike and fluctuation in levels of luteal progesterone), even small errors may impact analyses (challenges reviewed [16]). Further, whereas pregnancy mediates TDLU expansion, postpartum involution and age-related involution reduce TDLU content and re-shape the breast through different mechanisms.
Data related to measured circulating factors and TDLU involution are sparse, and based largely on visual assessment, morphometry or broad categorical classifications [3, 23, 24]. Previously, we evaluated associations between the circulating factors above and three visually assessed validated features of BC risk in normal breast tissues of 238 premenopausal donors to the Komen Tissue Bank (KTB): TDLU numbers, TDLU span, and acini per TDLU, assessed visually as a categorical variable [23, 24]. Our results differed by menopausal status for sex-steroid hormone levels. Among premenopausal women, we observed that levels of estradiol, free estradiol, free testosterone and progesterone were inversely associated with TDLU counts, whereas among postmenopausal women, estradiol and testosterone were positively associated with TDLU counts [23]. Prolactin was positively associated with TDLU counts among both premenopausal and postmenopausal women [23]. We also reported that higher IGF-1: IGFBP-3 ratios among premenopausal white women were linked to increased TDLU counts and that higher postmenopausal IGFBP-3 was related to lower TDLU counts among women of both races [24]. These data are consistent with associations of hormone levels and premenopausal BC risk as reported in some, but not in all studies [15, 20–22, 25–28].
Given that our prior analysis was limited by imprecision inherent in visual assessment and categorization rather than continuous quantitation of features [24, 29], we re-evaluated relationships of hormones and premenopausal breast histology using a newly developed and validated pathology Artificial Intelligence (AI) method, which provide automated, quantitative analysis of multiple breast morphologic features [29]. Herein, we assess relationships of previously measured serum hormone and growth factor measurements with 11 quantitative pathology AI features to assess relationships overall, and preliminarily with respect to modification by parity and other factors.
Methods
Participants
The KTB is a unique resource that enrolls and acquires blood and normal breast tissue samples from healthy volunteer subjects [30]. We analyzed breast tissues from 316 premenopausal KTB donors (44 parous and 272 nulliparous) enrolled from 2009–2018 with previously measured serum levels of estrogen, progesterone, testosterone, sex-steroid hormone binding globulin (SHBG), IGF-1 and related IGFBP-3, as described in detail elsewhere and available through the KTB [23]. The current analysis employed different inclusion and exclusion criteria than in the earlier publication, hence sample sizes differ. Herein, we excluded 28 women whose serum and tissue donations were >90 days after the first day of their last period. Data from previously completed self-administered questionnaires were evaluated, including age at donation, parity, ethnicity, race, body mass index (BMI, expressed as Kg/m2), current smoking, current drinking, age at first period, relative with breast/ovarian cancer, and number of days that have passed since first day of last period. Among parous women, we also assessed: number of live births, time since last birth, age at first birth, history of breastfeeding, and total months breastfeeding.
Pathology AI Analysis of Histology
Previously, we developed and validated automated pathology AI methods for analysis of benign breast tissues, with particular attention to assessment of lobules [29]. We applied this method to premenopausal KTB samples included herein. Features collected across the entire tissue section included TDLU count and adipose tissue fraction; features assessed per lobule were specified as: acini count, dilated acini, acini area, capillary area, epithelial area, epithelial-stromal ratio, mononuclear cell count, proximal adipose tissue area, and TDLU area. For TDLU level AI outcomes where there were multiple values for each subject, the mean value was calculated for each subject and used in statistical analysis.
Continuous variables were summarized as median (or mean), minimum, and maximum. Categorical variables were summarized as numbers and percentages. Associations of hormone measures and IGF-1, IGF-1/IGFBP-3 molar ratio with AI outcomes were examined using negative binomial regression models for count AI outcome variables (TDLU count, acini count, dilated acini, mononuclear cell count) and linear regression models for continuous AI outcome variables (adipose tissue fraction, acini area, capillary area, epithelial area, epithelial-stromal ratio, proximal adipose tissue area, and TDLU area). For negative binomial regression models, multiplicative effects on mean values of AI outcomes were estimated along with 95% confidence intervals. For linear regression models, natural logarithm and cube root data transformations of AI outcomes were applied to account for distributional skewness as needed (see table footnotes), and additive effects on the mean value of the given AI outcome were estimated along with 95% confidence intervals (CIs). Hormone measures, IGF-1, and IGF-1/IGGBP-3 molar ratios were considered on the natural logarithm scale in all regression analyses due to their skewed distributions.
Regression models were adjusted for pre-defined potential confounding variables as follows. Models were first adjusted for age at donation and parity alone, and then were subsequently additionally adjusted for BMI, percent fat in the tissue section, race, and days since the first day of last period (as both a linear and quadratic term). We assessed whether there were interactions between parity and measured serum analytes versus AI outcomes using multivariable linear regression models, adjusted for all of the aforementioned pre-defined potential confounding variables. We also tested interactions of testosterone with percent fat, BMI, and proximal adipose tissue area regarding associations with AI outcomes in multivariable linear regression analysis adjusting for these same potential confounding variables. We applied a Bonferroni correction for multiple testing separately for each group of similar statistical tests, after which p-values <0.0045 were considered as statistically significant given the 11 different AI outcomes that were evaluated. All statistical tests were two-sided and were performed using SAS (version 9.4; SAS Institute, Inc., Cary, North Carolina).
Results
Characteristics of parous and nulliparous participants and donated breast tissues
In descriptive analysis (Table 1), parous women were older (35 versus 23 years) and heavier (BMI: 27.4 versus 24.5) than nulliparas and more often had a positive family history of breast or ovarian cancer (63.4% versus 48.2%). Compared with nulliparas, parous women had higher circulating median levels of estrogen and lower levels of progesterone, prolactin, SHBG and testosterone.
Table 1:
Subject characteristics, hormone measures, and IGF-1/IGFBP-3 molar ratio
| Variable | N | All women (N=316) | N | Parous women (N=44) | N | Nulliparous women (N=272) |
|---|---|---|---|---|---|---|
| Age at donation (years) | 316 | 25 (21, 33) | 44 | 35 (32, 40) | 272 | 23 (21, 30) |
| Ethnicity (Hispanic/Latino) | 315 | 9 (2.9%) | 44 | 1 (2.3%) | 271 | 8 (3.0%) |
| Race | 316 | 44 | 272 | |||
| Caucasian | 282 (89.2%) | 37 (84.1%) | 245 (90.1%) | |||
| African American | 34 (10.8%) | 7 (15.9%) | 27 (9.9%) | |||
| BMI | 316 | 24.9 (22.0, 29.7) | 44 | 27.4 (22.7, 33.3) | 272 | 24.5 (22.0, 29.1) |
| Percent fat | 316 | 60 (40, 90) | 44 | 70 (50, 90) | 272 | 60 (40, 80) |
| Current smoking | 316 | 13 (4.1%) | 44 | 0 (0.0%) | 272 | 13 (4.8%) |
| Current drinking | 315 | 233 (74.0%) | 44 | 30 (68.2%) | 271 | 203 (74.9%) |
| Age at first period (years) | 316 | 12.5 (12, 13) | 44 | 13 (12, 13) | 272 | 12 (12, 13) |
| Number of live births | 44 | 44 | N/A | N/A | ||
| 1 | 17 (38.6%) | 17 (38.6%) | ||||
| 2 | 19 (43.2%) | 19 (43.2%) | ||||
| 3 | 7 (15.9%) | 7 (15.9%) | ||||
| 4 | 0 (0.0%) | 0 (0.0%) | ||||
| 5 | 1 (2.3%) | 1 (2.3%) | ||||
| Time since last birth (years) | 44 | 3.7 (2.4, 7.0) | 44 | 3.7 (2.4, 7.0) | N/A | N/A |
| Age at first birth (years) | 44 | 29 (26, 32) | 44 | 29 (26, 32) | N/A | N/A |
| History of breastfeeding | 44 | 37 (84.1%) | 44 | 37 (84.1%) | N/A | N/A |
| Total months breastfeeding | 37 | 12 (6, 19) | 37 | 12 (6, 19) | N/A | N/A |
| Total months breastfeeding (with no breastfeeding considered to be 0 months) | 44 | 7 (3, 18) | 44 | 7 (3, 18) | N/A | N/A |
| Relative with breast/ovarian cancer | 298 | 150 (50.3%) | 41 | 26 (63.4%) | 257 | 124 (48.2%) |
| Estrogen | 174 | 36 (10, 61) | 22 | 54 (45, 64) | 152 | 30 (9, 60) |
| Progesterone | 176 | 0.40 (0.20, 0.70) | 22 | 0.25 (0.20, 0.40) | 154 | 0.40 (0.20, 0.70) |
| Prolactin | 176 | 7.95 (6.10, 10.45) | 22 | 7.05 (5.80, 10.40) | 154 | 8.10 (6.20, 10.50) |
| SHBG | 149 | 91 (47, 167) | 20 | 80 (38, 115) | 129 | 93 (52, 191) |
| Testosterone | 172 | 16 (11, 27) | 21 | 11 (7, 20) | 151 | 17 (12, 29) |
| IGF-1 | 205 | 173 (135, 222) | 37 | 150 (126, 180) | 168 | 182 (140, 230) |
| IGF-1/IGFBP-3 molar ratio | 205 | 0.12 (0.10, 0.16) | 37 | 0.12 (0.10, 0.14) | 168 | 0.13 (0.10, 0.16) |
The sample median (Q1, Q3) is given for continuous variables.
In further descriptive analyses (Table 2), parous women had higher TDLU counts and capillary area, and greater proximity of TDLUs to adipose tissue, but lower acini area and smaller TDLU and epithelial areas. The ratio of epithelial to stromal area was lower in parous women.
Table 2:
AI outcome measures
| AI outcome measure | N | All women (N=316) | N | Parous women (N=44) | N | Nulliparous women (N=272) |
|---|---|---|---|---|---|---|
| Slide level | ||||||
| TDLU count | 316 | 10 (4, 22) | 44 | 12 (4, 25) | 272 | 10 (4, 21) |
| Adipose tissue fraction | 316 | 0.47 (0.23, 0.67) | 44 | 0.53 (0.34, 0.75) | 272 | 0.46 (0.21, 0.66) |
| TDLU level | ||||||
| Acini count | 305 | 20 (11, 34) | 44 | 19 (12, 32) | 261 | 21 (11, 34) |
| Dilated acini | 305 | 1 (0, 2) | 44 | 1 (0, 2) | 261 | 1 (0, 2) |
| Acini area | 305 | 2152 (1573, 3126) | 44 | 1860 (1384, 2704) | 261 | 2201 (1610, 3170) |
| Capillary area | 305 | 8073 (5272, 11527) | 44 | 8678 (5373, 12138) | 261 | 8073 (5272, 11242) |
| Epithelial area | 305 | 41342 (27126, 63100) | 44 | 31636 (23218, 58829) | 261 | 44117 (28422, 63924) |
| Epithelial:Stromal | 305 | 0.41 (0.33, 0.47) | 44 | 0.34 (0.29, 0.41) | 261 | 0.41 (0.34, 0.48) |
| Mononuclear cell count | 305 | 79 (49, 112) | 44 | 80 (59, 140) | 261 | 79 (49, 110) |
| Proximal adipose tissue area | 305 | 45130 (17552, 125326) | 44 | 64588 (19284, 163792) | 261 | 44743 (17359, 122573) |
| TDLU area | 305 | 132940 (85534, 192711) | 44 | 127868 (82649, 199735) | 261 | 134515 (85781, 191728) |
The sample median (Q1, Q3) is given.
Association of hormone levels and AI pathology features
Compared with other circulating factors analyzed, prolactin showed the greatest number of significant associations with AI features. In full multivariable analyses (adjusted for age, parity, BMI, percentage fat in the tissue section, race, and days since the first day of the last period), higher prolactin levels were related to increased proximity of adipose tissue to TDLUs (p<0.001) and greater TDLU area (p<0.001), with similar nominally significant (P<0.05) associations noted for TDLU counts (p=0.007), acini counts (p=0.012), dilated acini (p=0.047), capillary area (p=0.015), epithelial area (p=0.007), and mononuclear cell count (p=0.017) (Table 3). SHBG levels showed marginally significant associations with TDLU counts (p=0.035) and capillary area (p=0.052) in full multivariable analyses, but these findings were not statistically significant when correcting for multiple comparisons (Supplemental Table 1). Levels of testosterone were associated with increased TDLU counts in full multivariable analyses adjusting for confounding variables (p<0.001, Supplemental Table 2). Levels of estrogen, progesterone, IGF-1/IGFBP-3 molar ratio, and IGF-1 did not show statistically significant associations with histologic features in full multivariable analysis (Supplemental Tables 3-6). We performed sensitivity analyses in which we excluded 56 women who reported using oral contraceptives but associations between hormone levels and pathology AI features remained similar.
Table 3:
Associations between prolactin and AI outcome measures
| Association between prolactin (as a continuous variable) and the given AI outcome measure | |||||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Prolactin ≤ median (N=88) | Prolactin > median (N=88) | Adjusting for age and parity | Adjusting for age, parity, BMI, percent fat, race, days since first day of last period | ||||||
|
| |||||||||
| Outcome | N | Median (Q1, Q3) | N | Median (Q1, Q3) | Association measure | Estimate (95% CI) | P-value | Estimate (95% CI) | P-value |
| Slide level | |||||||||
| TDLU count1 | 88 | 10 (4, 22) | 88 | 17 (9, 35) | Multiplicative effect on mean | 1.66 (1.13, 2.43) | 0.009 | 1.67 (1.15, 2.43) | 0.007 |
| Adipose tissue fraction2 | 88 | 0.34 (0.10, 0.58) | 88 | 0.39 (0.17, 0.56) | Additive effect on mean | −0.03 (−0.12, 0.06) | 0.58 | −0.01 (−0.06, 0.05) | 0.83 |
| TDLU level | |||||||||
| Acini count1 | 84 | 18 (9, 32) | 86 | 25 (15, 41) | Multiplicative effect on mean | 1.40 (1.07, 1.84) | 0.016 | 1.42 (1.08, 1.86) | 0.012 |
| Dilated acini1 | 84 | 1 (0, 1) | 86 | 1 (0, 2) | Multiplicative effect on mean | 1.54 (1.01, 2.35) | 0.045 | 1.56 (1.01, 2.41) | 0.047 |
| Acini area2 | 84 | 2063 (1538, 3360) | 86 | 2003 (1540, 2853) | Additive effect on mean | −0.07 (−0.28, 0.14) | 0.53 | −0.07 (−0.28, 0.13) | 0.48 |
| Capillary area2 | 84 | 7303 (4801, 11536) | 86 | 9076 (5566, 11951) | Additive effect on mean | 0.35 (0.09, 0.61) | 0.009 | 0.34 (0.07, 0.60) | 0.015 |
| Epithelial area2 | 84 | 39467 (22642, 58492) | 86 | 50954 (32642, 72135) | Additive effect on mean | 0.37 (0.12, 0.62) | 0.004 | 0.36 (0.10, 0.61) | 0.007 |
| Epithelial:Stromal2 | 84 | 0.42 (0.33, 0.48) | 86 | 0.43 (0.36, 0.50) | Additive effect on mean | 0.00 (−0.04, 0.04) | 0.99 | −0.01 (−0.05, 0.03) | 0.77 |
| Mononuclear cell count1 | 84 | 69 (40, 96) | 86 | 86 (59, 118) | Multiplicative effect on mean | 1.30 (1.02, 1.65) | 0.033 | 1.34 (1.05, 1.71) | 0.017 |
| Proximal adipose tissue area2 | 84 | 26915 (9402, 73917) | 86 | 44147 (15422, 113587) | Additive effect on mean | 9.17 (2.95, 15.39) | 0.004 | 9.93 (4.11, 15.75) | <0.001 |
| TDLU area2 | 84 | 119854 (74788, 162052) | 86 | 151428 (96064, 202114) | Additive effect on mean | 0.43 (0.20, 0.66) | <0.001 | 0.45 (0.21, 0.69) | <0.001 |
Q1=first quartile; Q3=third quartile; CI=confidence interval. Days since first day of last period was adjusted for as both a linear and quadratic term.
Negative binomial regression models were used; multiplicative effects on the mean and 95% CIs were estimated and are interpreted as the multiplicative effect on the mean outcome value for each 1-unit increase in prolactin (on the natural logarithm scale).
Linear regression models were used; additive effects on the mean and 95% CIs were estimated and are interpreted as the additive effect on the mean outcome value (on the natural logarithm scale for acini area, capillary area, epithelial area, and TDLU area, and on the cube root scale for proximal adipose tissue area) for each 1-unit increase in prolactin (on the natural logarithm scale). P-values <0.0045 are considered as statistically significant after applying a Bonferroni correction for multiple testing.
Exploratory interaction analyses of relationships of hormones and AI pathology features by parity and other features
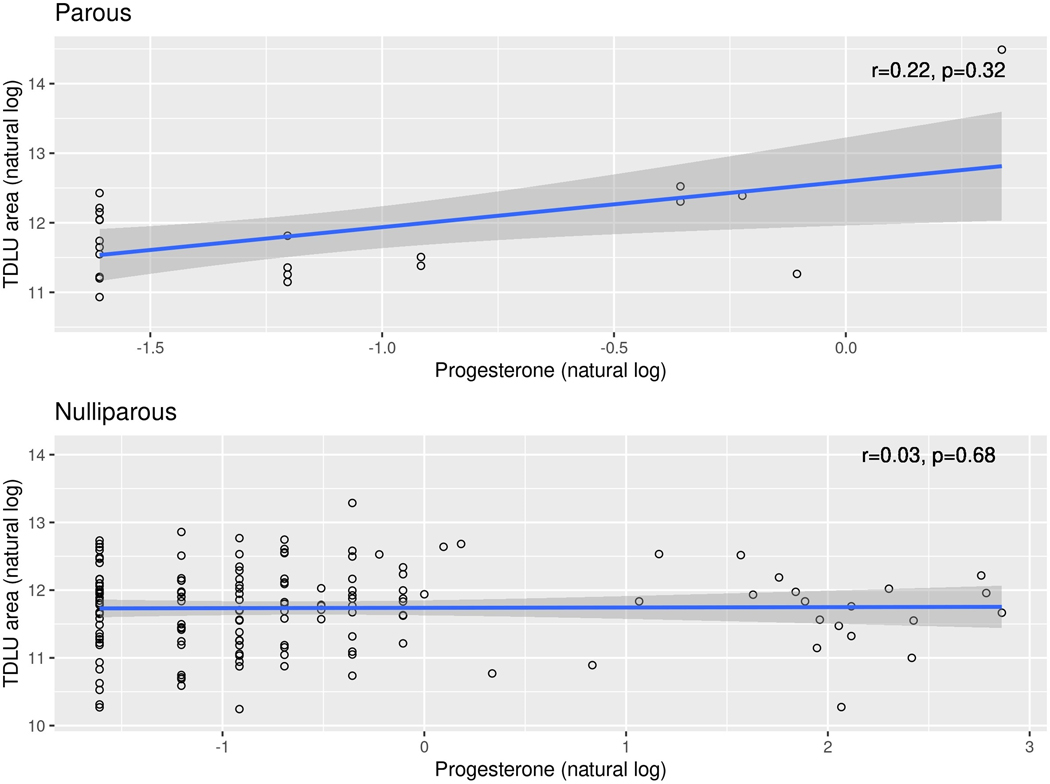
In exploratory multivariable interaction analyses, we first assessed whether associations between hormone levels and pathology AI features were modified by parity status. We observed a nominally significant interaction (p=0.032) between prolactin and parity regarding the association with acini area, with a stronger positive correlation for parous versus nulliparous women. We also noted a significant interaction (p=0.003, Figure 1) between progesterone and parity regarding association with TDLU area; although associations between progesterone and TDLU area in the separate parous and nulliparous groups themselves were not statistically significant, there was a stronger positive correlation for the parous compared to nulliparous women.
Figure 1:
Serum progesterone versus TDLU area, stratified by parity status
We next evaluated interactions of testosterone with percent fat, BMI, and proximal adipose tissue area regarding associations with AI features, and observed nominally significant interactions between testosterone and both acini counts (p=0.038; stronger positive correlation for women with a higher percent fat) and acini area (p=0.006; stronger negative correlation for women with a higher percent fat).
Discussion
Our analysis demonstrates significant inverse relationships between prolactin levels and several quantitative metrics of TDLU involution derived with AI methods in normal breast tissues of premenopausal women. Higher testosterone concentrations were related to higher TDLU counts, irrespective of parity, and SHBG showed marginally significant positive associations with TDLU counts. Further, preliminary explorations of limited statistical power suggest that relationships of hormone levels and breast histology may differ by parity status or fat content of the breast. These results confirm previously reported positive associations of prolactin with TDLU counts, as assessed visually among premenopausal women, providing additional confirmation of our AI methods and findings [23]; however, we did not confirm our prior finding that increased premenopausal estradiol and progesterone levels were linked to reduced TDLU counts (discussed below). These results extend our prior work by providing quantitation of additional histologic features in relation to hormone levels. Further, these data raise the hypothesis that the effect of circulating factors on BC risk may vary by parity and/or baseline breast histology.
In this analysis, increased prolactin levels were associated with higher levels of TDLU counts and acini counts, TDLU area, dilated acini, capillary area, epithelial area, and proximity to adipose tissue. Associations of prolactin levels with AI metrics were unaffected by adjustment for multiple covariates, including percentage of fat in tissues, a surrogate of mammographic density. Consistent with this result, a recent analysis found that circulating prolactin levels were not associated with premenopausal percent mammographic density, and also that prolactin and mammographic density measurements were related independently to increased premenopausal BC risk [20]. In another report, prolactin levels were linked to increased risk of postmenopausal BC, but unrelated to risk of premenopausal BC [21], although an earlier analysis in this cohort had suggested an association [19]. A meta-analysis of seven studies found an association of highest versus lowest circulating prolactin concentrations and increased BC risk for postmenopausal BC but not for premenopausal BC and for hormone receptor positive BC, but not for hormone receptor negative BC [31]. The positive association of prolactin levels and acini area in this analysis was stronger among parous versus nulliparous women, albeit based on a small sample size. Notably, women in this analysis were younger (median age 35 years), and parous women were more recently pregnant (median time since birth 3.7 years) than in published analyses of cohorts studying associations of circulating factors and BC risk.
The potential importance of prolactin in breast carcinogenesis remains controversial, despite the importance of this hormone in breast development and lactation (reviewed [32, 33]). Prolactin receptor is expressed in normal breast cells and BCs and prolactin signaling cooperates with several oncogenic protein kinases. In cell lines, prolactin contributes to increased proliferation and decreased apoptosis, suggesting the potential role of autocrine and paracrine signaling in the development of BC, and in preclinical models, sustained prolactin signaling is linked to BC development. Given that prolactin may be produced by breast cells and adipocytes, concentrations in breast tissues could exceed those in the blood. However, hyperprolactinemia (i.e., supraphysiological levels) has not been implicated in increased BC risk nor are mutations in prolactin or its receptors linked to BC, although a functional genetic variant in the receptor that mediates constitutive signaling has been identified.
Elegant preclinical studies suggest that during pregnancy and lactation epithelial cells bearing activated oncogenes may demonstrate aberrant persistent expression of prolactin receptor, which inhibits physiologic apoptosis during postpartum involution, and increases BC risk [34]. Thus, accumulation of mutant cells prior to pregnancy, levels of prolactin, derived from endocrine and intramammary tissues, and expression of prolactin receptor may interact to shape TDLU involution and potentially affect BC risk. The positive association of prolactin with BC risk is supported by experimental studies demonstrating that prolactin is related to increases in stem/progenitor cells and that crosstalk between prolactin and sex-steroid hormone signaling may contribute to cancer development in model systems [18]. We propose that evaluating the structure and function of normal breast tissues and BC precursors in conjunction with measurement of serum factors might improve BC risk assessment.
In our study, we found that testosterone levels were positively associated with number of TDLUs, regardless of parity; however, the mechanisms mediating this association are unclear. Increased testosterone blood levels have been linked to increased BC risk in some, but not all studies of premenopausal women, and more convincingly among postmenopausal women [15, 26]. In vitro, androgens reduce proliferation of ER-positive BC cells, but androgens also represent starting materials for estrogen synthesis, which increases postmenopausal BC risk [35]. Data suggest that necrotic adipocytes surrounded by macrophages, referred to as crown-like structures, may be linked to increased aromatization [36, 37] and elevated risk of incident BC [38]. Thus, the stronger positive association of testosterone with TDLU counts and stronger negative association with acini area may represent an effect of intracrinology (i.e., hormone metabolism within the breast). Androgen receptor is expressed in normal breast (except during late pregnancy and lactation) and in all BC molecular subtypes, with variably reported impact on prognosis and treatment responses [39].
The impact of progesterone on normal breast tissues and BC risk also remains controversial, with inconsistencies between mechanistic studies, which suggest an increase in BC risk, and epidemiological studies, which are generally null [27]. Further, both estrogen and progesterone are converted to numerous metabolites, with potentially different effects on risk and varying correlations of breast tissue and blood levels [28, 40, 41]. Our data suggest the potential value of examining these associations further, stratified by parity status, and possibly time since last birth, a factor which is not always collected and analyzed in etiological studies.
Although estrogen, progesterone and IGF levels were not linked to AI histology features in this study, these analyses were limited by statistical power and potential limitations in extrapolating menstrual cycle dates from dates of last menstrual period, particularly among women who do not have regular 28-day cycles (see above). In contrast to our prior analysis using visual review and a different set of KTB samples, we did not find inverse associations with estradiol and progesterone levels and TDLU counts, nor did we confirm marginally significant positive associations of IGF-1:IGFBP-3 with TDLU counts. Herein, we found strongest associations for prolactin and testosterone levels, factors which do not vary substantially with menstrual dates, raising the possibility that adjusting for last menstrual period is problematic when assessing hormone levels among cycling premenopausal women. Further, a small breast sample may not always represent the status of normal breast tissue. Given that histologic features likely integrate effects of multiple factors produced within the breast or at other organ sites over prolonged periods, breast histology may offer a useful biomarker of BC risk, especially among the more than one million women biopsied in the U.S. annually. Further, as use of digital pathology expands, the ability to apply AI methods in routine diagnostic practice will increase [42].
In conclusion, our analysis of normal breast tissues of premenopausal women provides strong evidence that prolactin alters features of TDLUs, with additional potential effects of testosterone. Larger studies are needed to understand the hormonal etiology of early onset BCs, particularly the role of prolactin, and to develop improved risk prediction and prevention strategies.
Supplementary Material
Acknowledgments and Funding Information
The authors thank donors to the Komen Tissue Bank. This work is supported in part by the Mayo Clinic Cancer Center P30CA15083-45.
Footnotes
Statement and Declarations
The authors declare no competing interests.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request or from the Komen Tissue Bank and Indiana University.
References
- 1.Wellings SR, Jensen HM, Marcum RG. An atlas of subgross pathology of the human breast with special reference to possible precancerous lesions. J Natl Cancer Inst. 1975;55(2):231–73. [PubMed] [Google Scholar]
- 2.Wallace TR, Tarullo SE, Crump LS, Lyons TR. Studies of postpartum mammary gland involution reveal novel pro-metastatic mechanisms. J Cancer Metastasis Treat. 2019;5. 10.20517/2394-4722.2019.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jindal S, Gao D, Bell P, Albrektsen G, Edgerton SM, Ambrosone CB, et al. Postpartum breast involution reveals regression of secretory lobules mediated by tissue-remodeling. Breast Cancer Res. 2014;16(2):R31. 10.1186/bcr3633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martinson HA, Jindal S, Durand-Rougely C, Borges VF, Schedin P. Wound healing-like immune program facilitates postpartum mammary gland involution and tumor progression. Int J Cancer. 2015;136(8):1803–13. 10.1002/ijc.29181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schedin P, O’Brien J, Rudolph M, Stein T, Borges V. Microenvironment of the involuting mammary gland mediates mammary cancer progression. J Mammary Gland Biol Neoplasia. 2007;12(1):71–82. 10.1007/s10911-007-9039-3. [DOI] [PubMed] [Google Scholar]
- 6.Lyons TR, O’Brien J, Borges VF, Conklin MW, Keely PJ, Eliceiri KW, et al. Postpartum mammary gland involution drives progression of ductal carcinoma in situ through collagen and COX-2. Nat Med. 2011;17(9):1109–15. 10.1038/nm.2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Faupel-Badger JM, Arcaro KF, Balkam JJ, Eliassen AH, Hassiotou F, Lebrilla CB, et al. Postpartum remodeling, lactation, and breast cancer risk: summary of a National Cancer Institute-sponsored workshop. J Natl Cancer Inst. 2013;105(3):166–74. 10.1093/jnci/djs505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Milanese TR, Hartmann LC, Sellers TA, Frost MH, Vierkant RA, Maloney SD, et al. Age-related lobular involution and risk of breast cancer. J Natl Cancer Inst. 2006;98(22):1600–7. 10.1093/jnci/djj439. [DOI] [PubMed] [Google Scholar]
- 9.Figueroa JD, Pfeiffer RM, Brinton LA, Palakal MM, Degnim AC, Radisky D, et al. Standardized measures of lobular involution and subsequent breast cancer risk among women with benign breast disease: a nested case-control study. Breast Cancer Res Treat. 2016;159(1):163–72. 10.1007/s10549-016-3908-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Figueroa JD, Pfeiffer RM, Patel DA, Linville L, Brinton LA, Gierach GL, et al. Terminal duct lobular unit involution of the normal breast: implications for breast cancer etiology. J Natl Cancer Inst. 2014;106(10). 10.1093/jnci/dju286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kensler KH, Liu EZF, Wetstein SC, Onken AM, Luffman CI, Baker GM, et al. Automated Quantitative Measures of Terminal Duct Lobular Unit Involution and Breast Cancer Risk. Cancer Epidemiol Biomarkers Prev. 2020;29(11):2358–68. 10.1158/1055-9965.EPI-20-0723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baer HJ, Collins LC, Connolly JL, Colditz GA, Schnitt SJ, Tamimi RM. Lobule type and subsequent breast cancer risk: results from the Nurses’ Health Studies. Cancer. 2009;115(7):1404–11. 10.1002/cncr.24167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Santucci-Pereira J, Zeleniuch-Jacquotte A, Afanasyeva Y, Zhong H, Slifker M, Peri S, et al. Genomic signature of parity in the breast of premenopausal women. Breast Cancer Res. 2019;21(1):46. 10.1186/s13058-019-1128-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nichols HB, Schoemaker MJ, Cai J, Xu J, Wright LB, Brook MN, et al. Breast Cancer Risk After Recent Childbirth: A Pooled Analysis of 15 Prospective Studies. Ann Intern Med. 2019;170(1):22–30. 10.7326/M18-1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Endogenous H, Breast Cancer Collaborative G, Key TJ, Appleby PN, Reeves GK, Travis RC, et al. Sex hormones and risk of breast cancer in premenopausal women: a collaborative reanalysis of individual participant data from seven prospective studies. Lancet Oncol. 2013;14(10):1009–19. 10.1016/S1470-2045(13)70301-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tin Tin S, Reeves GK, Key TJ. Endogenous hormones and risk of invasive breast cancer in pre- and post-menopausal women: findings from the UK Biobank. Br J Cancer. 2021;125(1):126–34. 10.1038/s41416-021-01392-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Key T, Appleby P, Barnes I, Reeves G, Endogenous H, Breast Cancer Collaborative G. Endogenous sex hormones and breast cancer in postmenopausal women: reanalysis of nine prospective studies. J Natl Cancer Inst. 2002;94(8):606–16. 10.1093/jnci/94.8.606. [DOI] [PubMed] [Google Scholar]
- 18.O’Leary KA, Rugowski DE, Shea MP, Sullivan R, Moser AR, Schuler LA. Prolactin synergizes with canonical Wnt signals to drive development of ER+ mammary tumors via activation of the Notch pathway. Cancer Lett. 2021;503:231–9. 10.1016/j.canlet.2021.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tworoger SS, Eliassen AH, Sluss P, Hankinson SE. A prospective study of plasma prolactin concentrations and risk of premenopausal and postmenopausal breast cancer. J Clin Oncol. 2007;25(12):1482–8. 10.1200/JCO.2006.07.6356. [DOI] [PubMed] [Google Scholar]
- 20.Gabrielson M, Ubhayasekera K, Ek B, Andersson Franko M, Eriksson M, Czene K, et al. Inclusion of Plasma Prolactin Levels in Current Risk Prediction Models of Premenopausal and Postmenopausal Breast Cancer. JNCI Cancer Spectr. 2018;2(4):pky055. 10.1093/jncics/pky055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tworoger SS, Eliassen AH, Zhang X, Qian J, Sluss PM, Rosner BA, et al. A 20-year prospective study of plasma prolactin as a risk marker of breast cancer development. Cancer Res. 2013;73(15):4810–9. 10.1158/0008-5472.CAN-13-0665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Endogenous H, Breast Cancer Collaborative G, Key TJ, Appleby PN, Reeves GK, Roddam AW. Insulin-like growth factor 1 (IGF1), IGF binding protein 3 (IGFBP3), and breast cancer risk: pooled individual data analysis of 17 prospective studies. Lancet Oncol. 2010;11(6):530–42. 10.1016/S1470-2045(10)70095-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khodr ZG, Sherman ME, Pfeiffer RM, Gierach GL, Brinton LA, Falk RT, et al. Circulating sex hormones and terminal duct lobular unit involution of the normal breast. Cancer Epidemiol Biomarkers Prev. 2014;23(12):2765–73. 10.1158/1055-9965.EPI-14-0667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oh H, Pfeiffer RM, Falk RT, Horne HN, Xiang J, Pollak M, et al. Serum insulin-like growth factor (IGF)-I and IGF binding protein-3 in relation to terminal duct lobular unit involution of the normal breast in Caucasian and African American women: The Susan G. Komen Tissue Bank. Int J Cancer. 2018;143(3):496–507. 10.1002/ijc.31333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eliassen AH, Tworoger SS, Hankinson SE. Reproductive factors and family history of breast cancer in relation to plasma prolactin levels in premenopausal and postmenopausal women. Int J Cancer. 2007;120(7):1536–41. 10.1002/ijc.22482. [DOI] [PubMed] [Google Scholar]
- 26.Hankinson SE, Eliassen AH. Circulating sex steroids and breast cancer risk in premenopausal women. Horm Cancer. 2010;1(1):2–10. 10.1007/s12672-009-0003-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Trabert B, Sherman ME, Kannan N, Stanczyk FZ. Progesterone and Breast Cancer. Endocr Rev. 2020;41(2). 10.1210/endrev/bnz001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stanczyk FZ, Mathews BW, Sherman ME. Relationships of sex steroid hormone levels in benign and cancerous breast tissue and blood: A critical appraisal of current science. Steroids. 2015;99(Pt A):91–102. 10.1016/j.steroids.2014.12.011. [DOI] [PubMed] [Google Scholar]
- 29.de Bel T LG, Ogony J, Stallings-Mann M, Carter JM, Hilton T, Radisky DC, Vierkant RA, Broderick B, Hoskin TL, Winham SJ, Frost MH, Visscher DW, Allers T, Degnim AC, Sherman ME, van der Lakk JAWM Automated quantiification of levels of breast terminal duct lobular (TDLU) involution using deep learning. NPJ Breast Cancer (In Press). 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sherman ME, Figueroa JD, Henry JE, Clare SE, Rufenbarger C, Storniolo AM. The Susan G. Komen for the Cure Tissue Bank at the IU Simon Cancer Center: a unique resource for defining the “molecular histology” of the breast. Cancer Prev Res (Phila). 2012;5(4):528–35. 10.1158/1940-6207.CAPR-11-0234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang M, Wu X, Chai F, Zhang Y, Jiang J. Plasma prolactin and breast cancer risk: a meta- analysis. Sci Rep. 2016;6:25998. 10.1038/srep25998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bernichtein S, Touraine P, Goffin V. New concepts in prolactin biology. J Endocrinol. 2010;206(1):1–11. 10.1677/JOE-10-0069. [DOI] [PubMed] [Google Scholar]
- 33.Goffin V Prolactin receptor targeting in breast and prostate cancers: New insights into an old challenge. Pharmacol Ther. 2017;179:111–26. 10.1016/j.pharmthera.2017.05.009. [DOI] [PubMed] [Google Scholar]
- 34.Haricharan S, Dong J, Hein S, Reddy JP, Du Z, Toneff M, et al. Mechanism and preclinical prevention of increased breast cancer risk caused by pregnancy. Elife. 2013;2:e00996. 10.7554/eLife.00996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Secreto G, Girombelli A, Krogh V. Androgen excess in breast cancer development: implications for prevention and treatment. Endocr Relat Cancer. 2019;26(2):R81–R94. 10.1530/ERC-18-0429. [DOI] [PubMed] [Google Scholar]
- 36.Morris PG, Hudis CA, Giri D, Morrow M, Falcone DJ, Zhou XK, et al. Inflammation and increased aromatase expression occur in the breast tissue of obese women with breast cancer. Cancer Prev Res (Phila). 2011;4(7):1021–9. 10.1158/1940-6207.CAPR-11-0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mullooly M, Yang HP, Falk RT, Nyante SJ, Cora R, Pfeiffer RM, et al. Relationship between crown-like structures and sex-steroid hormones in breast adipose tissue and serum among postmenopausal breast cancer patients. Breast Cancer Res. 2017;19(1):8. 10.1186/s13058-016-0791-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Carter JM, Hoskin TL, Pena MA, Brahmbhatt R, Winham SJ, Frost MH, et al. Macrophagic “Crown-like Structures” Are Associated with an Increased Risk of Breast Cancer in Benign Breast Disease. Cancer Prev Res (Phila). 2018;11(2):113–9. 10.1158/1940-6207.CAPR-17-0245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen M, Yang Y, Xu K, Li L, Huang J, Qiu F. Androgen Receptor in Breast Cancer: From Bench to Bedside. Front Endocrinol (Lausanne). 2020;11:573. 10.3389/fendo.2020.00573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Oh H, Khodr ZG, Sherman ME, Palakal M, Pfeiffer RM, Linville L, et al. Relation of Serum Estrogen Metabolites with Terminal Duct Lobular Unit Involution Among Women Undergoing Diagnostic Image-Guided Breast Biopsy. Horm Cancer. 2016;7(5–6):305–15. 10.1007/s12672-016-0265-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Falk RT, Brinton LA, Dorgan JF, Fuhrman BJ, Veenstra TD, Xu X, et al. Relationship of serum estrogens and estrogen metabolites to postmenopausal breast cancer risk: a nested case-control study. Breast Cancer Res. 2013;15(2):R34. 10.1186/bcr3416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.van der Laak J, Litjens G, Ciompi F. Deep learning in histopathology: the path to the clinic. Nat Med. 2021;27(5):775–84. 10.1038/s41591-021-01343-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request or from the Komen Tissue Bank and Indiana University.