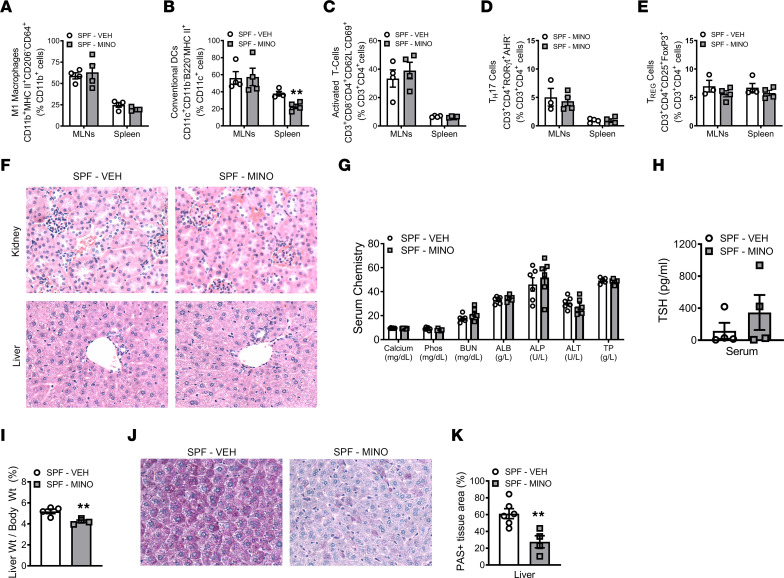
Figure 3. Minocycline does not alter gastrointestinal immunity or cause cytotoxic effects in kidney or liver.
Male C57BL/6T specific pathogen–free (SPF) mice were administered vehicle control (VEH) or minocycline (MINO) from age 6 to 12 weeks; euthanized at age 12 weeks. (A–E) Flow cytometric analysis in spleen and mesenteric lymph node (MLN) cells. (A) CD11b+MHCII+CD206–CD64+ M1 macrophages, reported as % CD11b+ cells; n = 4/group. (B) CD11c+CD11b–B220–MHCII+ conventional dendritic cells (DCs); reported as % CD11c+ cells; n = 4/group. (C) CD3+CD8−CD4+CD62L−CD69+ activated T cells; reported as % CD3+CD4+ cells; n = 4/group. (D) CD3+CD4+RORγt+AHR− TH17 cells and (E) CD3+CD4+CD25+FoxP3+ TREG cells; reported as % CD3+CD4+ cells; n = 3–4/group. (F) Representative H&E-stained kidney and liver sections used for histopathological evaluation (original magnification, 200×). (G) Serum chemistry analysis: calcium, phosphorus (Phos), blood urea nitrogen (BUN), albumin (ALB), alkaline phosphatase (ALP), alanine aminotransferase (ALT), total protein (TP); n = 6/group. (H) Thyroid-stimulating hormone (TSH) serum ELISA; n = 4/group. (I) Liver weight per body weight; n = 4/group. Periodic acid–Schiff–stained (PAS-stained) median liver lobe sections; n = 4–6/group: (J) representative images (original magnification, 200×), (K) PAS+ area per tissue area (%). Unpaired 2-tailed t test; reported as mean ± SEM; **P < 0.01 vs. VEH.