In the United States (US) as of December 2022, children represent 22% of the total population and comprise 17.2% of total accumulated COVID-19 cases.1,2 Contrary to other respiratory viruses, when compared to adults, infants and young children typically experience milder symptoms when infected with SARS-CoV-2.3 In this issue, Roversi et al. demonstrate that among a cohort of Italian children, older age, the lack of fever and respiratory symptoms, and the existence of other symptoms predicted a lower viral load. The findings of this study suggest that a lower viral load and clinical presentation are correlated among pediatric patients, which may be related to overall clinical course.
Despite milder symptoms, children continue to face a growing list of physical, mental, and social consequences due to the ramifications of the COVID-19 pandemic. Children and adolescents aged 0–17 years account for more than 1900 COVID-19-related deaths in the US.2 Of those children infected, up to 10% required hospitalization with a small percentage resulting in long-term sequelae, including multi-system inflammatory syndrome (MIS-C) and “Long COVID”.4 More than 9100 COVID-19-related MIS-C cases have been reported in children with 74 resulting in death.2 In addition to these medical consequences, from a mental health perspective, adolescents are experiencing higher rates of anxiety and depression due to the social implications of the pandemic.5,6 As research into the effects of the pandemic continues to expand, this includes the impacts experienced by children and adolescents extending beyond measurable outcomes of physical and mental health.7,8
In June 2022, the Federal Drug Administration authorized emergency use of both Moderna and Pfizer-BioNTech’s mRNA COVID-19 vaccines in children as young as 6 months of age. Both vaccines have been proven safe and effective in preventing SARS-CoV-2 infection and serious illness.9,10 Regarding recently emerging strains, BNT162b2 (Pfizer-BioNTech) is up to 60.1% effective among children 5–11 years old and up to 59.5% effective in adolescents 12–15 years old at preventing symptomatic Omicron-variant infections.2,11 In addition, BNT162b2 is up to 93% effective in preventing hospitalizations and up to 96% effective in preventing critical illness due to the Delta variant in adolescents 12–18 years.2,10
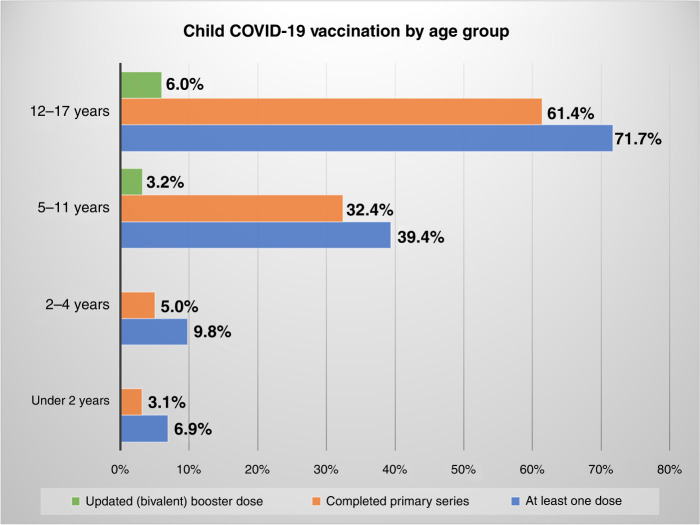
Despite the effectiveness of COVID-19 vaccines, only 68.9% of the US population age 5 years and older has completed a primary series, defined as two doses of an mRNA vaccine or one single-dose COVID-19 vaccine.2,12 Of those who have completed a primary series, only 65.1% have received a bivalent booster and are considered up-to-date regarding vaccination status.2,13 By age group, only 3.0% of children less than 2 years old, 4.9% of children 2–4 years old, 32.3% of adolescents 5–11 years old, and 61.4% of adolescents 12–17 years old have completed a primary series.2 Figure 1 highlights the vaccination rates by age group and number of doses received.2,12 In contrast to older age groups within the total US population, vaccination rates among children and adolescents are the lowest.2,12
Fig. 1. Child COVID-19 vaccination by age group.
Bar chart of percentage of COVID-19 vaccinations by age group determined by the total count of vaccinations within each age group devided by United States Census Bureau estimates for total population within age each group. The green bar represents the percentage of children who have completed the primary COVID-19 vaccine series and received an updated (bivalent booster dose). The orange bar represents the percentage of children with a completed primary series, defined as those who have received the second dose of the 2 dose COVID-19 vaccine series. The blue bar represents the percentage of children who have received at least one dose of COVID-19 vaccine series.2
There are clear demographic disparities in vaccination status. For example, although disparities by race and ethnicity are largely absent for having received at least one dose, among children 5–17 years who have completed a primary series, an estimated 16% of White, non-Hispanic individuals have received a bivalent booster compared to 10.1% of Black, non-Hispanic and 8.3% of Hispanic individuals.2 Only 29.1% of uninsured children 6 months to 17 years have received at least one dose compared to 42.9% with non-Medicaid insurance.2 In rural areas, only 25.4% of children have received at least one dose compared to 40.5% of those in urban areas.2 Historically, Black, Hispanic, low-income, and rural populations have exhibited lower vaccination rates, which is often attributed to barriers to access, lack of information, and lack of trust regarding vaccine safety.14,15 More recently, increasing disbursement of misinformation via traditional and social media platforms, and rising political influence have augmented vaccine hesitancy.16 The combination of socioeconomic barriers, vaccine hesitancy, misinformation, and the resulting lower vaccination rates among children present complex challenges in protecting children and adolescents from the multifaceted effects of the COVID-19 pandemic.
COVID-19 testing and treatment availability are also continuing problems for many individuals. The Health Resources and Services Administration (HRSA) expansion of the COVID-19 Testing Supply Program made at-home tests and rapid antigen tests available at health centers and Medicaid-certified rural health clinics in an attempt to reach populations living in poverty, racial and ethnic minorities, and other groups with the greatest risk for severe COVID-19-related outcomes.17 The Biden Administration made at-home rapid antigen tests available once again via mail delivery for a limited round of ordering beginning December 15, 2022.18 Still, despite efforts to increase testing accessibility, racial and ethnic minorities, and individuals in rural areas remain underrepresented in COVID-19 testing data throughout the US.19
The future lapse in federal government funding for COVID-19 testing, treatment, and vaccines will have widespread consequences that will disproportionately impact uninsured and underserved individuals.20 The HRSA COVID-19 program, which reimbursed the cost of services provided to uninsured individuals, relied on funds from the federal government and subsequently stopped accepting claims on April 5, 2022. While some 15 states still allow expanded Medicaid coverage to cover costs of COVID-19 testing, treatment, and vaccines for uninsured individuals, that coverage will end once the Department of Health and Human Services fails to renew the official Public Health Emergency (PHE). Although individuals with insurance coverage currently are not responsible for cost-sharing related to COVID-19 testing and vaccines during the PHE under federal law, additional barriers to access will likely arise once the PHE ends.
The federal government previously purchased a limited supply of vaccines prior to the termination of funding and an additional 171 million bivalent booster doses; however, that limited supply would not be sufficient to ensure up-to-date coverage for the entire US population.21,22 Only 3.2% of children 5–11 years old and 6.0% of children 12–17 years old have received a bivalent dose with an estimated 149 million doses already distributed.2 Although the cost of providing vaccines to uninsured individuals will no longer be reimbursed, providers are required to offer vaccines to all individuals at no cost under current federal law. To compensate for the additional financial burden, some providers may resort to billing patients for other COVID-19-related services. For smaller practices, providing adequate reimbursement rates will ensure these practices continue to provide the COVID-19 vaccine. In addition, given the rapid expiration of multi-dose vials, an important cost consideration, providing access to single-dose vials for pediatric providers must be a priority. Safety net providers, which continue to offer services at no cost, will undoubtedly see the steepest increase in financial burden due to the cease of federally funded reimbursements. While individuals with full Medicaid coverage will continue to have access to tests and treatments throughout the PHE, and for one year after, privately insured individuals, including those from the Affordable Care Act (ACA) Marketplace, may face cost-sharing and limited access to services without a prescription once the PHE ends. However, because the CDC Advisory Committee on Immunization Practices (ACIP) has recommended the vaccine, commercial payers must provide coverage for the vaccine without cost-sharing under the ACA.
Once the federally funded supply of tests, treatments, and vaccines is exhausted, uninsured individuals will face the biggest barriers to access and will most likely be required to pay out-of-pocket for all services. As illustrated by current and historical vaccination rates, uninsured children will see a disproportionate increase in barriers preventing access to future COVID-19 vaccines. Uninsured individuals without a primary care physician will also be at a greater disadvantage in accessing services that require a prescription once the PHE ends. Uninsured children and racial and ethnic minority children, who already exhibit some of the lowest vaccination rates, will experience a further exacerbation in disparities related to vaccine access. Therefore, the remaining federal supply should be prioritized for these vulnerable groups. US insurance companies and healthcare providers will also begin ordering tests, treatments, and vaccines from manufacturers on an as-needed basis resulting in diminishing supplies and rising costs as demand increases. Therefore, it will be important to continue working with manufacturers to ensure the supply chain remains active and functional.
The ACIP’s recommendation to add the COVID-19 vaccine to the Vaccines for Children (VFC) program and child/adolescent routine immunization schedules is an important first step in ensuring access to the vaccine.23 However, considering current vaccination rates among children and adolescents, securing sufficient funds for all children to receive primary doses and additional doses each year should remain a priority. The VFC program should be expanded to allow providers to administer vaccines to all underinsured children, instead of referring those children to a Federally Qualified Health Center or Rural Health Clinic.24 In addition, the VFC regional maximum charges should be increased to allow adequate Medicaid coverage of vaccine administration costs. Further steps to reduce financial and logistical barriers faced by patients and providers must be explored and implemented. Children and adolescents will continue to face the impacts of the pandemic and COVID-19 until equitable vaccine access can be ensured regardless of insurance and socioeconomic status.
Author contributions
Substantial contributions to conception and design: K.K., K.M. Drafting the article: K.K., K.M. Revising the article critically for important intellectual content: A.M.-F., C.G. Final approval of the version to be published: K.K., A.M.-F., C.G., K.M.
Data availability
The datasets analyzed during the current study are available in the Centers for Disease Control and Prevention COVID Data Tracker repository, https://covid.cdc.gov/covid-data-tracker.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
A list of authors and their affiliations appears at the end of the paper.
Contributor Information
Kimberly Montez, Email: kmontez@wakehealth.edu.
On behalf of the Pediatric Policy Council:
Shetal Shah, Jean Raphael, Mona Patel, David Keller, Lisa Chamberlain, Tina Cheng, Sherin Devaskar, Joyce Javier, and Lois Lee
References
- 1.National KIDS COUNT. Total Population by Child and Adult Populations in the United States (accessed 26 September 2022); https://datacenter.kidscount.org/data/tables/99-total-population-by-child-and-adult-populations#detailed/1/any/false/574,1729,37,871,870,573,869,36,868,867/39,40,41/416,417 (2021).
- 2.Centers for Disease Control. COVID Data Tracker (accessed 20 December 2022); https://covid.cdc.gov/covid-data-tracker (2022).
- 3.Zimmermann P, Curtis N. Why is COVID-19 less severe in children? A review of the proposed mechanisms underlying the age-related difference in severity of SARS-CoV-2 infections. Arch. Dis. Child. 2021;106:429–439. doi: 10.1136/archdischild-2020-320338. [DOI] [PubMed] [Google Scholar]
- 4.Howard‐Jones AR, et al. COVID-19 in children. II: pathogenesis, disease spectrum and management. J. Paediatr. Child Health. 2022;58:46–53. doi: 10.1111/jpc.15811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jones EAK, Mitra AK, Bhuiyan AR. Impact of COVID-19 on mental health in adolescents: a systematic review. Int. J. Environ. Res. Public Health. 2021;18:2470. doi: 10.3390/ijerph18052470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nearchou F, Flinn C, Niland R, Subramaniam SS, Hennessy E. Exploring the impact of COVID-19 on mental health outcomes in children and adolescents: a systematic review. Int. J. Environ. Res. Public Health. 2020;17:8479. doi: 10.3390/ijerph17228479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheng TL, Moon M, Artman M. Shoring up the safety net for children in the COVID-19 pandemic. Pediatr. Res. 2020;88:349–351. doi: 10.1038/s41390-020-1071-7. [DOI] [PubMed] [Google Scholar]
- 8.De St. Maurice A, et al. Pediatrician’s role in vaccinating children and families for COVID-19: no one left behind. Pediatr. Res. 2021;90:1105–1107. doi: 10.1038/s41390-021-01804-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Polack FP, et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N. Engl. J. Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Price AM, et al. BNT162b2 protection against the omicron variant in children and adolescents. N. Engl. J. Med. 2022;386:1899–1909. doi: 10.1056/NEJMoa2202826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fleming-Dutra KE, et al. Association of prior BNT162b2 COVID-19 vaccination with symptomatic SARS-CoV-2 infection in children and adolescents during Omicron predominance. JAMA. 2022;327:2210. doi: 10.1001/jama.2022.7493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.USA Facts. Us Coronavirus Vaccine Tracker (accessed 17 September 2022); https://usafacts.org/visualizations/covid-vaccine-tracker-states (2022).
- 13.Centers for Disease Control and Prevention. COVID-19 Vaccination (accessed 20 December 2022); https://www.cdc.gov/coronavirus/2019-ncov/vaccines/stay-up-to-date.html, https://www.cdc.gov/coronavirus/2019-ncov/vaccines/stay-up-to-date.html?s_cid=11747:cdc%20up%20to%20date%20vaccination:sem.ga:p:RG:GM:gen:PTN:FY22 (2022).
- 14.Ayers CK, et al. Disparities in H1n1 vaccination rates: a systematic review and evidence synthesis to inform COVID-19 vaccination efforts. J. Gen. Intern. Med. 2021;36:1734–1745. doi: 10.1007/s11606-021-06715-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hudson A, Montelpare WJ. Predictors of vaccine hesitancy: implications for COVID-19 public health messaging. Int. J. Environ. Res. Public Health. 2021;18:8054. doi: 10.3390/ijerph18158054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Finney Rutten LJ, et al. Evidence-based strategies for clinical organizations to address COVID-19 vaccine hesitancy. Mayo Clin. Proc. 2021;96:699–707. doi: 10.1016/j.mayocp.2020.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Health Resources & Services Administration. Expanding Access to COVID-19 Testing Supplies | HRSA (accessed September 2022); https://www.hrsa.gov/coronavirus/testing-supplies#testing.
- 18.The White House. Fact Sheet: Biden Administration Announces COVID-19 Winter Preparedness Plan (accessed 21 December 2022); https://www.whitehouse.gov/briefing-room/statements-releases/2022/12/15/fact-sheet-biden-administration-announces-covid-19-winter-preparedness-plan/ (2022).
- 19.Lyu, J., Cui, W. & Finkelstein, J. Assessing disparities in COVID-19 testing using National Covid Cohort Collaborative. Stud. Health Technol. Inform.295, 316–319 (2022). [DOI] [PMC free article] [PubMed]
- 20.Tolbert, J., Artiga, S., Kates, J. & Rudowitz, R. Implications of the Lapse in Federal COVID-19 Funding on Access to COVID-19 Testing, Treatment, and Vaccines (accessed 13 September 2022); https://www.kff.org/coronavirus-covid-19/issue-brief/implications-of-the-lapse-in-federal-covid-19-funding-on-access-to-covid-19-testing-treatment-and-vaccines/ (2022).
- 21.Kates, J., Michaud, J. & Levitt, L. Are There Enough COVID-19 Vaccines for America without More Funding? (accessed 13 September 2022); https://www.kff.org/coronavirus-covid-19/issue-brief/are-there-enough-covid-19-vaccines-for-america-without-more-funding/ (2022).
- 22.Kates, J., Cox, C. & Michaud, J. How Much Could COVID-19 Vaccines Cost the U.S. after Commercialization? (accessed 20 December 2022); https://www.kff.org/coronavirus-covid-19/issue-brief/how-much-could-covid-19-vaccines-cost-the-u-s-after-commercialization/ (2022).
- 23.Wilson, S. Acip Recommends Adding COVID-19 Vaccination to All Schedules (accessed December 2022); https://www.aafp.org/news/health-of-the-public/acip-oct-mtg.html.
- 24.Centers for Disease Control and Prevention. About the Vaccines for Children Program (VFC) (accessed 20 December 2022); https://www.cdc.gov/vaccines/programs/vfc/about/index.html (2021).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets analyzed during the current study are available in the Centers for Disease Control and Prevention COVID Data Tracker repository, https://covid.cdc.gov/covid-data-tracker.