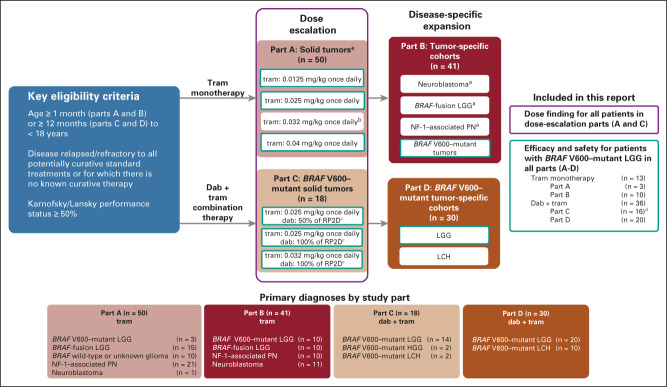
FIG 1.
Study design. aCohort not restricted to patients with BRAF V600–mutant disease. bIntermediate dose added on the basis of steady state pharmacokinetics and the tolerability of 0.025 mg/kg and 0.04 mg/kg once-daily doses. cPreviously determined RP2D for dabrafenib monotherapy7: age < 12 years, 5.25 mg/kg; age ≥ 12 years, 4.5 mg/kg, divided into two equal doses daily. dTwo patients with BRAF V600–mutant HGG were enrolled in part C and are included in this predominantly LGG cohort as a diagnosis of HGG did not define any other disease-specific cohort in the trial. dab, dabrafenib; HGG, high-grade glioma; LCH, Langerhans cell histiocytosis; LGG, low-grade glioma; NF-1, neurofibromatosis type 1; PN, plexiform neurofibroma; RP2D, recommended phase II dose; tram, trametinib.