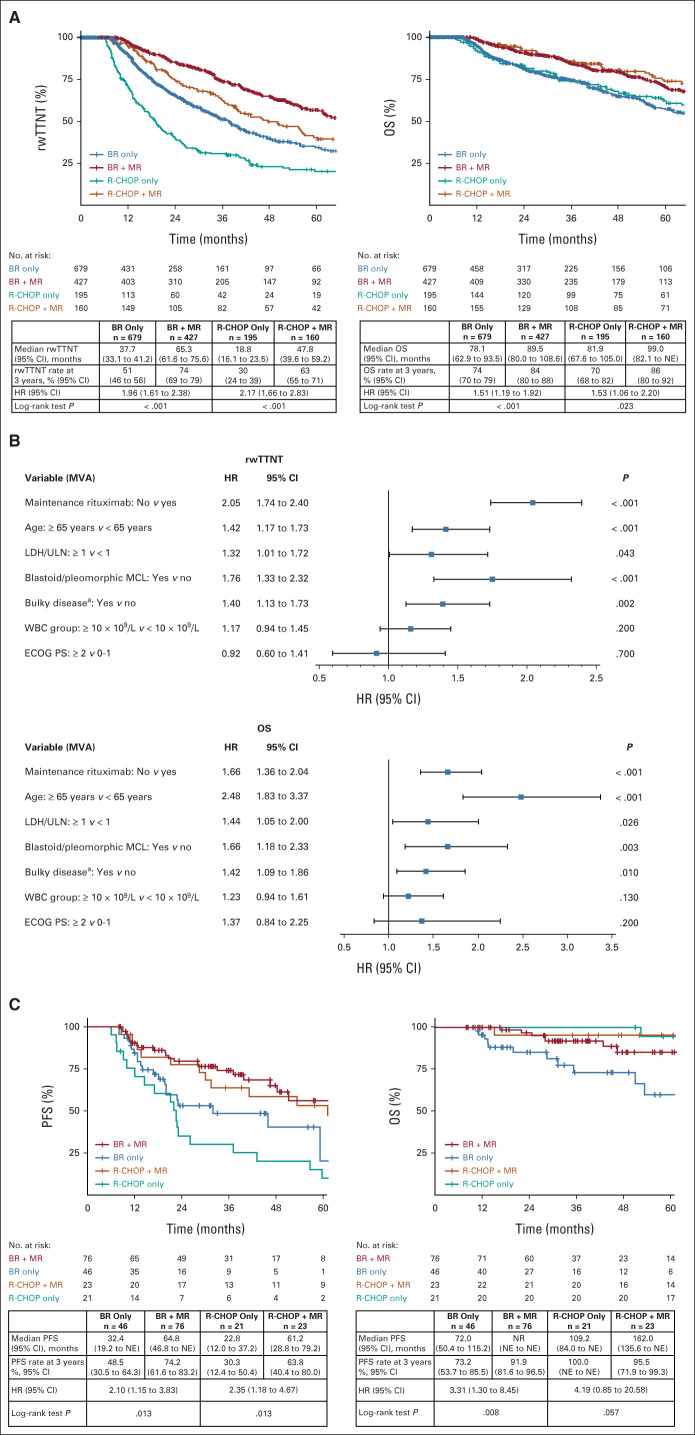
FIG 4.
rwTTNT and OS in the MR-eligible cohort: (A) patients treated with BR or R-CHOP alone, or BR + MR and R-CHOP + MR from the Flatiron cohort; (B) MVA of the predictors of rwTTNT and OS in the MR-eligible cohort from the Flatiron cohort; the MVA used all data, including missing values, as a category for covariates; (C) PFS and OS in the validation cohort. HR was calculated for MR yes versus no in the Flatiron cohort and MR no versus yes in the validation cohort. Median OS should be interpreted with caution as it was reached when few patients were still at risk. a“Bulky” as per local oncologist definition. BR, bendamustine plus rituximab; ECOG PS, Eastern Cooperative Oncology Group performance status; HR, hazard ratio; LDH, lactate dehydrogenase; MCL, mantle cell lymphoma; MR, maintenance rituximab; MVA, multivariate analysis; NE, not estimable; NR, not reached; OS, overall survival; PFS, progression-free survival; R-CHOP, rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone; rwTTNT, real-world time to next treatment; ULN, upper limit of normal.