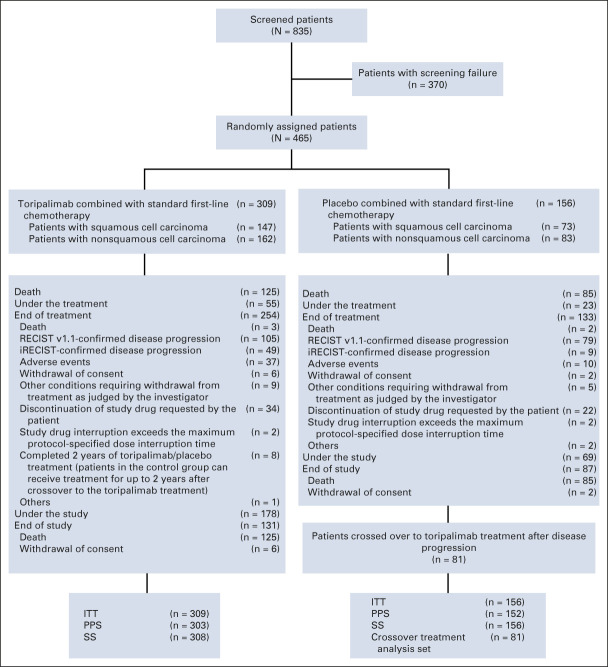
FIG 1.
CONSORT diagram of the CHOICE-01 study. From April 2, 2019, to August 5, 2020, a total of 835 patients were screened. Of these, 370/835 patients (44.3%) failed screening, mainly because of inclusion/exclusion criteria not met (305/370; 82.4%) or withdrawal of the informed consent (57/370; 15.4%). A total of 465 patients were successfully screened and randomly assigned 2:1 to the toripalimab plus chemotherapy arm (n = 309) or placebo plus chemotherapy arm (n = 156), stratified by PD-L1 expression status, histology (squamous v nonsquamous), and smoking status. By the cutoff date, 55 (17.8%) patients in the toripalimab arm and 23 (14.7%) in the placebo arm remained on the study treatment. ITT, intention-to-treat; PD-L1, programmed death ligand-1; PPS, per protocol set; SS, safety set.