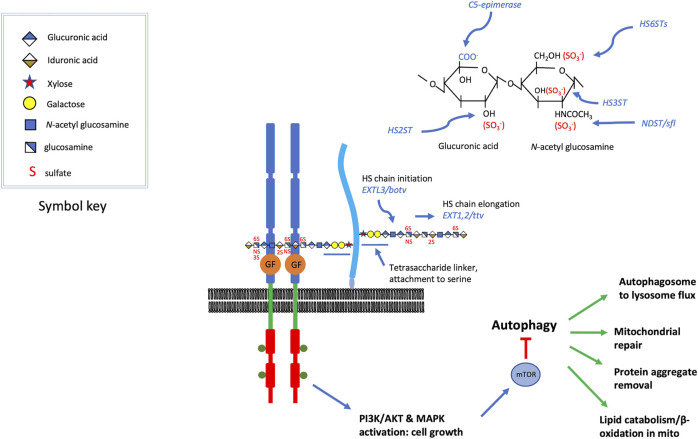
FIGURE 1.
HSPGs serve as growth factor co-receptors. For a variety of secreted growth factors, HSPGs provide co-receptor function (Hassan et al., 2020), promoting the binding of the growth factor to the signaling receptor (the figure is roughly modeled on FGF, FGFR assembly) (syndecans are transmembrane and glypicans bear a GPI-linkage, which is depicted here). The heparan sulfate chains are critical for the assembly of these signaling complexes and some of the key genes affecting heparan sulfate modification are shown. These include, O-linked sulfotransferases, an epimerase, and an N-deacetylase N-sulfotransferase. Heparan sulfate chain initiation, added to the non-reducing end of a tetrasaccharide linker attached to specific serine residues of the core protein, requires the activity of the glycosyltransferase encoded by EXTL3 or brother of tout-velu (botv) in humans and Drosophila, respectively. Chain elongation is achieved by the activity of enzymes encoded by EXT1/2 or tout velu (ttv) and sister of tout velu (sotv), in humans and Drosophila respectively (non-reducing end toward polymer terminus). Signaling mediated by many of the growth factors affected by heparan sulfate co-receptors activate PI3 kinase or MAPK, inputs that regulate Target of Rapamycin (TOR) activity. These growth promoting functions also serve to suppress catabolic activity, in large measure via mTOR inhibition of autophagy. Some of the cellular functions of autophagy are listed, and these are suppressed when growth factor signaling is high. The elevation of autophagy and catabolic activity occurs when heparan sulfate biosynthesis is compromised, reducing the activity of the growth factors for which they serve as co-receptors.